Page 647 - Williams Hematology ( PDFDrive )
P. 647
622 Part VI: The Erythrocyte Chapter 42: Iron Metabolism 623
80
Regulation of Hepcidin by Erythropoiesis but other cytokines including activin B may also contribute. Chronic
Intestinal iron absorption is increased severalfold after hemorrhage inflammation impairs iron supply to erythropoiesis and combines with
or erythropoietin administration, and is chronically increased in other effects of inflammation to cause anemia of inflammation (anemia
patients with ineffective erythropoiesis but not in aplastic anemia. of chronic disease, see Chap. 37).
73
These observations led to the hypothesis that the marrow generates an
“erythroid regulator” that modulates intestinal iron absorption. Later
73
studies in mouse models provided evidence that the erythroid reg- TRANSPORT OF IRON
56
ulator is a marrow-derived suppressor of hepcidin. Erythroferrone is
an erythropoietin-induced erythroblast-secreted glycoprotein that acts Once an atom of iron enters the blood plasma from dietary iron absorp-
on hepatocytes to suppress their hepcidin production and is required tion, it is virtually trapped in the body (Fig. 42–4) and cycles almost
for rapid suppression of hepcidin after hemorrhage or erythropoietin endlessly from the plasma to the developing erythroblast (where it is
administration. It also contributes to hepcidin suppression and iron used in hemoglobin synthesis), thence into the circulating blood for
74
overload in murine models of β-thalassemia intermedia. Growth dif- approximately 4 months, and then to macrophages. Here it is removed
ferentiation factor 15 (GDF15), a member of the BMP family, may also from heme by heme oxygenase and released back into the plasma to
contribute to pathologic hepcidin suppression in anemias with ineffec- repeat the cycle.
tive erythropoiesis. 75 The major function of the transport protein transferrin is to move
iron from wherever it enters the plasma (intestinal villi, splenic and
hepatic sinusoids) to the erythroblasts of the marrow and to other sites
Regulation of Hepcidin by Inflammation of use.
Within hours after the onset of systemic infection, plasma iron concen-
tration decreases. The response is thought to contribute to host defense,
particularly against microbes with high dependence on environmental ENDOCYTOSIS OF TRANSFERRIN
iron. This response, hypoferremia of inflammation, is also triggered Diferric (holo)transferrin binds to the transferrin receptor (TfR)-1 on
76
by noninfectious causes of acute and chronic inflammation. Hypofer- the cell surface and the holotransferrin–TfR1 complex forms clusters
remia of inflammation is mediated by cytokine-induced increase in in pits on the cell membrane. The complex is then internalized by
81
plasma hepcidin concentrations causing hepcidin-induced sequestra- endocytosis (Fig. 42–5). Within the cytosol the holotransferrin-TfR1
54
tion of iron in macrophages. The main human cytokine responsible for complex is in a clathrin-coated vesicle. The vesicles fuse with endoso-
hepcidin induction is IL-6 52,53 acting via the JAK2-STAT3 pathway, 77–79 mes and become acidified to pH 5 which releases iron from transferrin.
Hepatocytes
Splenic and other
macrophages
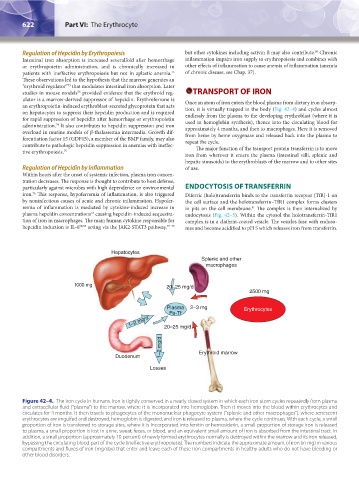
1000 mg 20–25 mg/d
2500 mg
Plasma 2–3 mg Erythrocytes
Fe-Tf
1–2 mg/d 20–25 mg/d
1–2 mg/d
Duodenum Erythroid marrow
Losses
Figure 42–4. The iron cycle in humans. Iron is tightly conserved in a nearly closed system in which each iron atom cycles repeatedly from plasma
and extracellular fluid (“plasma”) to the marrow, where it is incorporated into hemoglobin. Then it moves into the blood within erythrocytes and
circulates for 4 months. It then travels to phagocytes of the mononuclear phagocyte system (“splenic and other macrophages”), where senescent
erythrocytes are engulfed and destroyed, hemoglobin is digested, and iron is released to plasma, where the cycle continues. With each cycle, a small
proportion of iron is transferred to storage sites, where it is incorporated into ferritin or hemosiderin, a small proportion of storage iron is released
to plasma, a small proportion is lost in urine, sweat, feces, or blood, and an equivalent small amount of iron is absorbed from the intestinal tract. In
addition, a small proportion (approximately 10 percent) of newly formed erythrocytes normally is destroyed within the marrow and its iron released,
bypassing the circulating blood part of the cycle (ineffective erythropoiesis). The numbers indicate the approximate amount of iron (in mg) in various
compartments and fluxes of iron (mg/day) that enter and leave each of these iron compartments in healthy adults who do not have bleeding or
other blood disorders.
Kaushansky_chapter 42_p0617-0626.indd 622 9/17/15 6:26 PM