Page 649 - Williams Hematology ( PDFDrive )
P. 649
624 Part VI: The Erythrocyte Chapter 42: Iron Metabolism 625
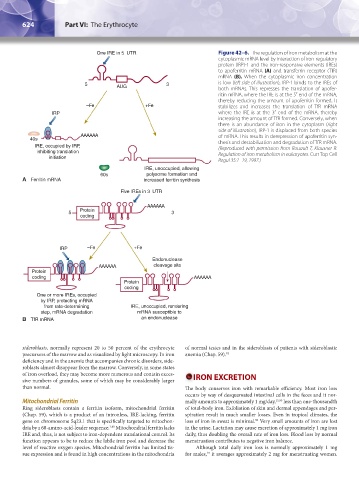
One IRE in 5 UTR Figure 42–6. The regulation of iron metabolism at the
cytoplasmic mRNA level by interaction of iron-regulatory
protein (IRP)-1 and the iron-responsive elements (IREs)
to apoferritin mRNA (A) and transferrin receptor (TfR)
mRNA (B). When the cytoplasmic iron concentration
5 AUG 3 is low (left side of illustration), IRP-1 binds to the IREs of
both mRNAs. This represses the translation of apofer-
ritin mRNA, where the IRE is at the 5′ end of the mRNA,
thereby reducing the amount of apoferritin formed. It
–Fe +Fe stabilizes and increases the translation of TfR mRNA
IRP where the IRE is at the 3′ end of the mRNA, thereby
increasing the amount of TfR formed. Conversely, when
there is an abundance of iron in the cytoplasm (right
side of illustration), IRP-1 is displaced from both species
AAAAAA of mRNA. This results in derepression of apoferritin syn-
40s thesis and destabilization and degradation of TfR mRNA.
IRE, occupied by IRP, (Reproduced with permission from Rouault T, Klausner R:
inhibiting translation Regulation of iron metabolism in eukaryotes. Curr Top Cell
initiation
Regul 35:1–19, 1997.)
IRE, unoccupied, allowing
60s polysome formation and
A Ferritin mRNA increased ferritin synthesis
Five IREs in 3 UTR
AAAAAA
5 Protein 3
coding
IRP –Fe +Fe
Endonuclease
AAAAAA cleavage site
Protein
coding AAAAAA
Protein
coding
One or more IREs, occupied
by IRP, protecting mRNA
from rate-determining IRE, unoccupied, rendering
step, mRNA degradation mRNA susceptible to
B TfR mRNA an endonuclease
sideroblasts, normally represent 20 to 50 percent of the erythrocyte of normal testes and in the sideroblasts of patients with sideroblastic
precursors of the marrow and as visualized by light microscopy. In iron anemia (Chap. 59). 92
deficiency and in the anemia that accompanies chronic disorders, side-
roblasts almost disappear from the marrow. Conversely, in some states
of iron overload, they may become more numerous and contain exces- IRON EXCRETION
sive numbers of granules, some of which may be considerably larger
than normal. The body conserves iron with remarkable efficiency. Most iron loss
occurs by way of desquamated intestinal cells in the feces and it nor-
Mitochondrial Ferritin mally amounts to approximately 1 mg/day, 15,93 less than one-thousandth
Ring sideroblasts contain a ferritin isoform, mitochondrial ferritin of total-body iron. Exfoliation of skin and dermal appendages and per-
(Chap. 59), which is a product of an intronless, IRE-lacking, ferritin spiration result in much smaller losses. Even in tropical climates, the
gene on chromosome 5q23.1 that is specifically targeted to mitochon- loss of iron in sweat is minimal. Very small amounts of iron are lost
90
dria by a 60-amino-acid-leader sequence. Mitochondrial ferritin lacks in the urine. Lactation may cause excretion of approximately 1 mg iron
1,91
IRE and, thus, is not subject to iron-dependent translational control. Its daily, thus doubling the overall rate of iron loss. Blood loss by normal
function appears to be to reduce the labile iron pool and decrease the menstruation contributes to negative iron balance.
level of reactive oxygen species. Mitochondrial ferritin has limited tis- Although total daily iron loss is normally approximately 1 mg
sue expression and is found in high concentrations in the mitochondria for males, it averages approximately 2 mg for menstruating women.
15
Kaushansky_chapter 42_p0617-0626.indd 624 9/17/15 6:26 PM