Page 1022 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1022
CHaPter 73 Sarcoidosis 985
A major conceptual challenge in sarcoidosis is understanding
how pulmonary fibrosis occurs in an environment dominated
by IFN-γ, which is known to inhibit collagen synthesis. Alter-
natively activated macrophages (M2) have been histologically
associated with myofibrosis in biopsy specimens of muscular
39
sarcoidosis. Although an M2 macrophage phenotype is often
associated with a Th2 cytokine environment (which has never
been identified in sarcoidosis, even in late fibrotic disease), a
fibrosis-promoting M2c macrophage phenotype could be induced
by IL-10 or CCL18, which are present in sarcoidosis tissues. 40,41
M2 macrophages can promote fibrocyte recruitment through
the expression of transforming growth factor (TGF), CCL18,
and CXCL12; M2 macrophages are also capable of differentiation
to fibrocyte-like cells that express collagen. It remains unclear
whether M2-like macrophages result from mechanisms inherent
to sarcoidosis or from generalized wound-healing responses.
Serum Amyloid A Aggregation Hypothesis
Although sarcoidosis is associated with prior exposure to myco-
bacterial or other microbial organisms, there is no clinical or
pathological evidence of active infection or reactivation of latent
microbial infection in sarcoidosis. This holds true in patients after
years of corticosteroid, immunosuppressive, or anti-TNF therapy.
The physicochemical properties of the Kveim reagent closely
resemble those of amyloid or prion proteins. Serum amyloid A
(SAA) can be detected in epithelioid macrophages in sarcoidosis.
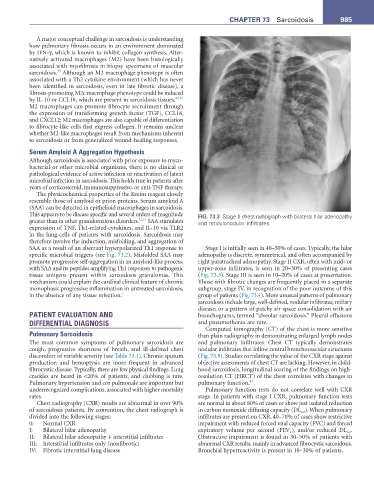
This appears to be disease specific and several orders of magnitude FIG. 73.3 Stage II chest radiograph with bilateral hilar adenopathy
greater than in other granulomatous disorders. 42,51 SAA stimulates and reticulonodular infiltrates.
expression of TNF, Th1-related cytokines, and IL-10 via TLR2
in the lung cells of patients with sarcoidosis. Sarcoidosis may
therefore involve the induction, misfolding, and aggregation of
SAA as a result of an aberrant hyperpolarized Th1 response to Stage I is initially seen in 40–50% of cases. Typically, the hilar
specific microbial triggers (see Fig. 73.2). Misfolded SAA may adenopathy is discrete, symmetrical, and often accompanied by
promote progressive self-aggregation in an amyloid-like process, right paratracheal adenopathy. Stage II CXR, often with mid- or
with SAA and its peptides amplifying Th1 responses to pathogenic upper-zone infiltrates, is seen in 20–30% of presenting cases
tissue antigens present within sarcoidosis granulomas. This (Fig. 73.3). Stage III is seen in 10–20% of cases at presentation.
mechanism could explain the cardinal clinical feature of chronic Those with fibrotic changes are frequently placed in a separate
monophasic progressive inflammation in untreated sarcoidosis, subgroup, stage IV, in recognition of the poor outcome of this
in the absence of any tissue infection. 1 group of patients (Fig. 73.4). More unusual patterns of pulmonary
sarcoidosis include large, well-defined, nodular infiltrates; miliary
disease; or a pattern of patchy air-space consolidation with air
PATIENT EVALUATION AND bronchograms, termed “alveolar sarcoidosis.” Pleural effusions
DIFFERENTIAL DIAGNOSIS and pneumothorax are rare.
Computed tomography (CT) of the chest is more sensitive
Pulmonary Sarcoidosis than plain radiography in demonstrating enlarged lymph nodes
The most common symptoms of pulmonary sarcoidosis are and pulmonary infiltrates. Chest CT typically demonstrates
cough, progressive shortness of breath, and ill-defined chest nodular infiltrates that follow central bronchovascular structures
discomfort of variable severity (see Table 73.1). Chronic sputum (Fig. 73.5). Studies correlating the value of the CXR stage against
production and hemoptysis are more frequent in advanced objective assessments of chest CT are lacking. However, in child-
fibrocystic disease. Typically, there are few physical findings. Lung hood sarcoidosis, longitudinal scoring of the findings on high-
crackles are heard in <20% of patients, and clubbing is rare. resolution CT (HRCT) of the chest correlates with changes in
Pulmonary hypertension and cor pulmonale are important but pulmonary function. 43
underrecognized complications, associated with higher mortality Pulmonary function tests do not correlate well with CXR
rates. stage. In patients with stage I CXR, pulmonary function tests
Chest radiography (CXR) results are abnormal in over 90% are normal in about 80% of cases or show just isolated reduction
of sarcoidosis patients. By convention, the chest radiograph is in carbon monoxide diffusing capacity (DL co ). When pulmonary
divided into the following stages: infiltrates are present on CXR, 40–70% of cases show restrictive
0: Normal CXR impairment with reduced forced vital capacity (FVC) and forced
I: Bilateral hilar adenopathy expiratory volume per second (FEV 1 ), and/or reduced DL co .
II: Bilateral hilar adenopathy + interstitial infiltrates Obstructive impairment is found in 30–50% of patients with
III: Interstitial infiltrates only (nonfibrotic) abnormal CXR results, mainly in advanced fibrocystic sarcoidosis.
IV: Fibrotic interstitial lung disease Bronchial hyperreactivity is present in 10–30% of patients.