Page 1021 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1021
984 Part seven Organ-Specific Inflammatory Disease
Genetic/epigenetic +
Genetic/epigenetic +
Mycobacterial infection
Mycobacterial infection environmental factors
environmental factors
+ +
1. Innate response 2. Induces hyperpolarized Th1
2. Induces hyperpolarized Th1
1. Innate response
response to pathogenic microbial Ags
induces systemic and response to pathogenic microbial Ags
induces systemic and
intracellular SAA and SAA misfolding/aggregation
intracellular SAA
and SAA misfolding/aggregation
SAA mKatG
SAA
mKatG
MHC TCR SAA receptor
MHC
TCR
SAA receptor
T T
APC
APC
IFNg
+ + TNFa IFNg ? ?
TNFa
+ +
Treg
IL10
IL10 Treg
3. Misfolded/aggregated SAA “seeds” 4. SAA induces feed-forward
3. Misfolded/aggregated SAA “seeds”
4. SAA induces feed-forward
further SAA accumulation and amplification of local Ag-specific
further SAA accumulation and
amplification of local Ag-specific
release of soluble SAA peptides Th1 responses to trapped Ags
release of soluble SAA peptides
Th1 responses to trapped Ags
Inability to clear
Inability to clear Clearance ofClearance of
SAA and Ags leads to chronic
SAA and Ags leads to chronic SAA and AgsSAA and Ags
inflammation and fibrosis allows remission
inflammation and fibrosis
allows remission
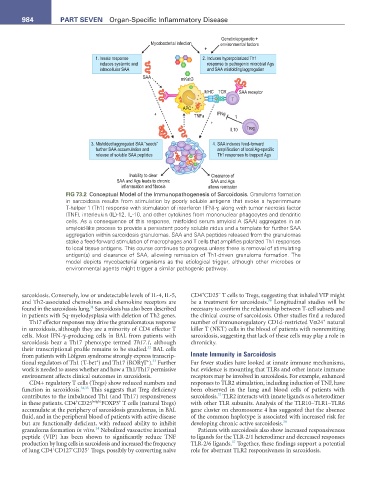
FIG 73.2 Conceptual Model of the Immunopathogenesis of Sarcoidosis. Granuloma formation
in sarcoidosis results from stimulation by poorly soluble antigens that evoke a hyperimmune
T-helper 1 (Th1) response with stimulation of interferon (IFN)-γ, along with tumor necrosis factor
(TNF), interleukin (IL)-12, IL-10, and other cytokines from mononuclear phagocytes and dendritic
cells. As a consequence of this response, misfolded serum amyloid A (SAA) aggregates in an
amyloid-like process to provide a persistent poorly soluble nidus and a template for further SAA
aggregation within sarcoidosis granulomas. SAA and SAA peptides released from the granulomas
stoke a feed-forward stimulation of macrophages and T cells that amplifies polarized Th1 responses
to local tissue antigens. This course continues to progress unless there is removal of stimulating
antigen(s) and clearance of SAA, allowing remission of Th1-driven granuloma formation. The
model depicts mycobacterial organisms as the etiological trigger, although other microbes or
environmental agents might trigger a similar pathogenic pathway.
−
+
sarcoidosis. Conversely, low or undetectable levels of IL-4, IL-5, CD4 CD25 T cells to Tregs, suggesting that inhaled VIP might
36
and Th2-associated chemokines and chemokine receptors are be a treatment for sarcoidosis. Longitudinal studies will be
31
found in the sarcoidosis lung. Sarcoidosis has also been described necessary to confirm the relationship between T-cell subsets and
in patients with 5q-myelodysplasia with deletion of Th2 genes. the clinical course of sarcoidosis. Other studies find a reduced
+
Th17 effector responses may drive the granulomatous response number of immunoregulatory CD1d-restricted Vα24 natural
in sarcoidosis, although they are a minority of CD4 effector T killer T (NKT) cells in the blood of patients with nonremitting
cells. Most IFN-γ–producing cells in BAL from patients with sarcoidosis, suggesting that lack of these cells may play a role in
sarcoidosis bear a Th17 phenotype termed Th17.1, although chronicity.
32
their transcriptional profile remains to be studied. BAL cells
from patients with Löfgren syndrome strongly express transcrip- Innate Immunity in Sarcoidosis
+
+
33
tional regulators of Th1 (T-bet ) and Th17 (RORγT ). Further Far fewer studies have looked at innate immune mechanisms,
work is needed to assess whether and how a Th1/Th17 permissive but evidence is mounting that TLRs and other innate immune
environment affects clinical outcomes in sarcoidosis. receptors may be involved in sarcoidosis. For example, enhanced
CD4+ regulatory T cells (Tregs) show reduced numbers and responses to TLR2 stimulation, including induction of TNF, have
function in sarcoidosis. 34,35 This suggests that Treg deficiency been observed in the lung and blood cells of patients with
37
contributes to the imbalanced Th1 (and Th17) responsiveness sarcoidosis. TLR2 interacts with innate ligands as a heterodimer
+
+
in these patients. CD4 CD25 bright FOXP3 T cells (natural Tregs) with other TLR subunits. Analysis of the TLR10–TLR1–TLR6
accumulate at the periphery of sarcoidosis granulomas, in BAL gene cluster on chromosome 4 has suggested that the absence
fluid, and in the peripheral blood of patients with active disease of the common haplotype is associated with increased risk for
but are functionally deficient, with reduced ability to inhibit developing chronic active sarcoidosis. 38
34
granuloma formation in vitro. Nebulized vasoactive intestinal Patients with sarcoidosis also show increased responsiveness
peptide (VIP) has been shown to significantly reduce TNF to ligands for the TLR-2/1 heterodimer and decreased responses
37
production by lung cells in sarcoidosis and increased the frequency TLR-2/6 ligands. Together, these findings support a potential
+
+
-
of lung CD4 CD127 CD25 Tregs, possibly by converting naïve role for aberrant TLR2 responsiveness in sarcoidosis.