Page 1257 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1257
1218 Part ten Prevention and Therapy of Immunological Diseases
many European countries publish their own country-specific computation, and science will result in improvements in human
immunization schedules, and vaccination guidelines that are health remains to be seen. At present there is some (healthy)
published by the WHO are utilized by many developing countries. skepticism about the big data systems biology approach. The
The schedules are generally similar but with some region-specific field must show that the massive data can be analyzed
differences. For example, the 2016 US ACIP immunization and integrated and that the approach is more than a fishing
schedule for children recommends vaccinations against 10 viral expedition but, rather, is an exploratory engine that is hypothesis
diseases: hepatitis B, rotavirus infection, polio, influenza, measles, generating and also leads to models that will be tested and will
58
mumps, rubella, varicella, hepatitis A, and HPV infection. produce new knowledge, resulting in improved vaccines that
Preventive viral vaccines in the WHO-recommended routine benefit human health.
immunization schedule for children include the same 10 viral For outstanding scientists to be attracted to and retained in
vaccines (although four of those, mumps, influenza, varicella, the field of vaccinology, it is essential that science funding agencies
and hepatitis A vaccines, are recommended only for country- provide and maintain robust support for critical discovery research
specific immunization programs with certain characteristics). projects (investigator-initiated research projects) that form the
The WHO schedule also recommends some additional vaccines engine of innovation that drives all of science. At the same time,
(e.g., rabies, yellow fever, Japanese encephalitis, and tick-borne targeted big science vaccine program projects and networks have
encephalitis vaccines are recommended for certain high-risk the potential to synergistically pool their approaches in an intense
populations). 59 focused effort to tackle major vaccine needs and challenges (e.g.,
development of HIV and TB vaccines). To translate basic advances
SOME PRESENT AND FUTURE CHALLENGES and laboratory science into improved health care for patients,
having well-trained translational physician-scientists lead
The science of vaccinology is exciting and strong, having entered clinical–translational human research programs is an additional
a new era with the high-throughput analytical and data approaches component of essential infrastructure. There is a need for
being applied today. Whether these advances in technology, postdoctoral training programs that focus on vaccinology, but
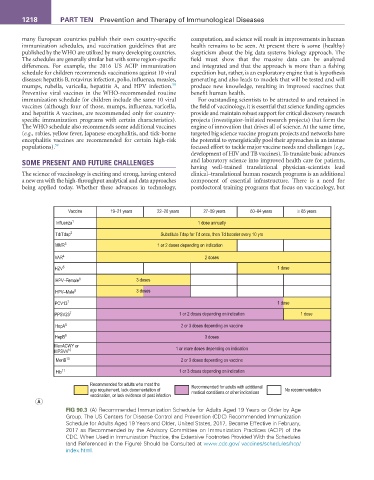
Vaccine 19–21 years 22–26 years 27–59 years 60–64 years ≥ 65 years
Influenza 1 1 dose annually
Td/Tdap 2 Substitute Tdap for Td once, then Td booster every 10 yrs
MMR 3 1 or 2 doses depending on indication
VAR 4 2 doses
HZV 5 1 dose
HPV–Female 6 3 doses
HPV–Male 6 3 doses
PCV13 7 1 dose
PPSV23 7 1 or 2 doses depending on indication 1 dose
HepA 8 2 or 3 doses depending on vaccine
HepB 9 3 doses
MenACWY or
MPSV4 10 1 or more doses depending on indication
MenB 10 2 or 3 doses depending on vaccine
Hib 11 1 or 3 doses depending on indication
Recommended for adults who meet the Recommended for adults with additional
age requirement, lack documentation of medical conditions or other indications No recommendation
vaccination, or lack evidence of past infection
A
FIG 90.3 (A) Recommended Immunization Schedule for Adults Aged 19 Years or Older by Age
Group. The US Centers for Disease Control and Prevention (CDC) Recommended Immunization
Schedule for Adults Aged 19 Years and Older, United States, 2017, Became Effective in February,
2017 as Recommended by the Advisory Committee on Immunization Practices (ACIP) of the
CDC. When Used in Immunization Practice, the Extensive Footnotes Provided With the Schedules
(and Referenced in the Figure) Should be Consulted at www.cdc.gov/ vaccines/schedules/hcp/
index.html.