Page 1259 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1259
1220 Part ten Prevention and Therapy of Immunological Diseases
Public awareness and education about vaccination must
be enhanced through professional marketing campaigns. The A Vaccine Against Human Immunodeficiency Virus
negative consequences of propaganda by antivaccination groups, The development of a vaccine against human immunodeficiency
alternative or delayed schedules of vaccination, and parental virus/acquired immunodeficiency syndrome (HIV/AIDS) has
hesitancy regarding vaccination can all result in resurgence of long been recognized as a top HIV research global priority at
infectious diseases, increased morbidity, and increased vaccine- the US National Institutes of Health (NIH). Strong and simple
preventable deaths. treatments for those who are living with HIV infection are now
With modern air travel, which makes it possible to go from in existence in the United States and have been made available
one continent to another in a matter of a few hours, it is no even in developing countries. It has been shown that the
stretch to say we dwell in a global village, where maintaining treatment-as-prevention approach, wherein the viral load is
good health and avoiding highly contagious infectious diseases lowered to an undetectable level by antiretroviral treatment of
depend on the health of communities, whether local or global. infected persons, results not only in benefit to the infected patient
Therefore it is in our self-interest to maintain high levels of but also in up to 96% reduction in HIV incidence among sexual
vaccination in our home communities and to advocate and partners. 60,61
support vaccination efforts around the globe. Clearly, vaccination More recently, antiretroviral drugs (ARDs) have been tested
is both a personal and a social, or community, endeavor. globally and licensed in the United States as a once-daily pill (a
A few specific challenges for vaccination are highlighted below combination of tenofovir and emtricitabine) for HIV/AIDS
62
to illustrate the general points made above. prevention in higher-risk individuals. Known as preexposure
13-15
11-12
Vaccine Birth 1 mo 2 mos 4 mos 6 mos 9 mos 12 mos 15 mos 18 mos 19-23 2-3 yrs 4-6 yrs 7-10 yrs 11-12 13-15 16 yrs 17-18
yrs
mos
yr
yrs
s
yrs
yr
s
nd
rd
st
1
Hepatitis B (HepB) 1 dose 2 dose 3 dose
2
Rotavirus ,(RV) RV1 (2-dose st nd See
series);RV5 (3-dose series) 1 dose 2 dose footnote 2
Diphtheria, tetanus, & acellular st nd rd th th
3
pertussis (DTaP: <7 yrs) 1 dose 2 dose 3 dose 4 dose 5 dose
th
rd
+DHPRSKLOXV LQIOXHQ]DH 1 dose 2 dose See 3 or 4 dose,
nd
st
4
type b (Hib) footnote 4 See footnote 4
Pneumococcal conjugate 5 st nd 3 dose 4 dose
rd
th
(PCV13) 1 dose 2 dose
Inactivated poliovirus 6 1 dose 2 dose 3 dose 4 dose
th
rd
nd
st
(IPV: < 18 yrs)
7 Annual vaccination (IIV)
Influenza (IIV) Annual vaccination (IIV) 1 or 2 doses
1 dose only
nd
8
st
Measles,mumps,rubella (MMR) See footnote 8 1 dose 2 dose
9
st
nd
Varicella (VAR) 1 dose 2 dose
10
HepatitisA (HepA) 2-dose series, See footnote 10
11
Meningococcal (Hib-MenCY
st
nd
≥6 weeks; MenACWY-D ≥9 mos; See footnote 11 1 dose 2 dose
MenACWY-CRM ≥2 mos)
Tetanus, diphtheria,& acellular Tdap
12
pertussis (Tdap: ≥7 yrs)
13
Human papillomavirus (HPV) See footnote
13
See footnote 11
Meningococcal B 14
Pneumococcal polysaccharide 15 See footnote 5
(PPSV23)
Range of recommended Range of recommended ages Range of recommended ages Range of recommended ages for non-high-risk No recommendation
ages for all children for catch-up immunization for certain high-risk groups groups that may receive vaccine subject to
A
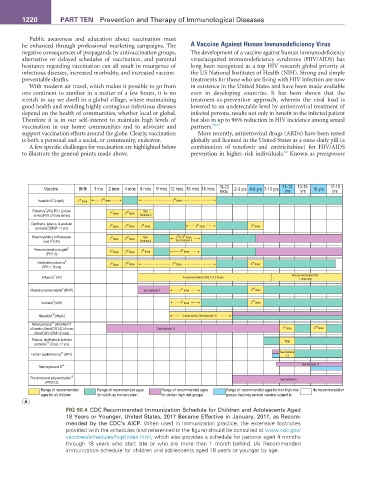
FIG 90.4 CDC Recommended Immunization Schedule for Children and Adolescents Aged
18 Years or Younger, United States, 2017 Became Effective in January, 2017, as Recom-
mended by the CDC’s AICP. When used in immunization practice, the extensive footnotes
provided with the schedules (and referenced in the figure) should be consulted at www.cdc.gov/
vaccines/schedules/hcp/index.html, which also provides a schedule for persons aged 4 months
through 18 years who start late or who are more than 1 month behind. (A) Recommended
immunization schedule for children and adolescents aged 18 years or younger by age.