Page 272 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 272
CHAPTER 17 Cytotoxic T Lymphocytes and Natural Killer Cells 253
CD56 bright CD56 dim
CCR7 CD16 dim CXCR1 CD16 bright
CXCR3 CX3CR1
CD62L
Perforin/ Perforin/
Granzymes Granzymes
+ +++
Cytokine
production
+
CD94-NKG2 KIR Cytokine CD94-NKG2 KIR
+++ +/- production + +++
+++
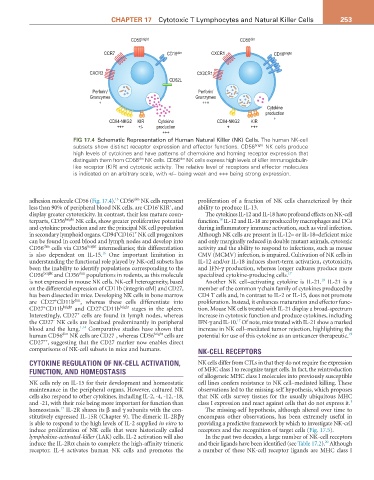
FIG 17.4 Schematic Representation of Human Natural Killer (NK) Cells. The human NK-cell
subsets show distinct receptor expression and effector functions. CD56 bright NK cells produce
high levels of cytokines and have patterns of chemokine and homing receptor expression that
dim
dim
distinguish them from CD56 NK cells. CD56 NK cells express high levels of killer immunoglobulin-
like receptor (KIR) and cytotoxic activity. The relative level of receptors and effector molecules
is indicated on an arbitrary scale, with +/− being weak and +++ being strong expression.
16
dim
adhesion molecule CD56 (Fig. 17.4). CD56 NK cells represent proliferation of a fraction of NK cells characterized by their
+
+
less than 90% of peripheral blood NK cells, are CD16 KIR , and ability to produce IL-13.
display greater cytotoxicity. In contrast, their less mature coun- The cytokines IL-12 and IL-18 have profound effects on NK-cell
18
terparts, CD56 bright NK cells, show greater proliferative potential function. IL-12 and IL-18 are produced by macrophages and DCs
and cytokine production and are the principal NK cell population during inflammatory immune activation, such as viral infection.
+
+
in secondary lymphoid organs. CD94 CD161 NK cell progenitors Although NK cells are present in IL-12– or IL-18–deficient mice
can be found in cord blood and lymph nodes and develop into and only marginally reduced in double mutant animals, cytotoxic
dim
CD56 cells via CD56 bright intermediaries; this differentiation activity and the ability to respond to infections, such as mouse
16
is also dependent on IL-15. One important limitation in CMV (MCMV) infection, is impaired. Cultivation of NK cells in
understanding the functional role played by NK-cell subsets has IL-12 and/or IL-18 induces short-term activation, cytotoxicity,
been the inability to identify populations corresponding to the and IFN-γ production, whereas longer cultures produce more
dim
CD56 bright and CD56 populations in rodents, as this molecule specialized cytokine-producing cells. 17
19
is not expressed in mouse NK cells. NK-cell heterogeneity, based Another NK cell–activating cytokine is IL-21. IL-21 is a
on the differential expression of CD11b (integrin αM) and CD27, member of the common γ chain family of cytokines produced by
has been dissected in mice. Developing NK cells in bone marrow CD4 T cells and, in contrast to IL-2 or IL-15, does not promote
+
dim
are CD27 CD11b , whereas these cells differentiate into proliferation. Instead, it enhances maturation and effector func-
+
-
CD27 CD11b bright and CD27 CD11b bright stages in the spleen. tion. Mouse NK cells treated with IL-21 display a broad-spectrum
+
Interestingly, CD27 cells are found in lymph nodes, whereas increase in cytotoxic function and produce cytokines, including
17
-
the CD27 NK cells are localized predominantly in peripheral IFN-γ and IL-10. Of note, mice treated with IL-21 show a marked
blood and the lung. 1,14 Comparative studies have shown that increase in NK cell–mediated tumor rejection, highlighting the
dim
–
human CD56 NK cells are CD27 , whereas CD56 bright cells are potential for use of this cytokine as an anticancer therapeutic. 19
++
CD27 , suggesting that the CD27 marker now enables direct
comparisons of NK-cell subsets in mice and humans. NK-CELL RECEPTORS
CYTOKINE REGULATION OF NK-CELL ACTIVATION, NK cells differ from CTLs in that they do not require the expression
FUNCTION, AND HOMEOSTASIS of MHC class I to recognize target cells. In fact, the reintroduction
of allogeneic MHC class I molecules into previously susceptible
NK cells rely on IL-15 for their development and homeostatic cell lines confers resistance to NK cell–mediated killing. These
maintenance in the peripheral organs. However, cultured NK observations led to the missing-self hypothesis, which proposes
cells also respond to other cytokines, including IL-2, -4, -12, -18, that NK cells survey tissues for the usually ubiquitous MHC
and -21, with their role being more important for function than class I expression and react against cells that do not express it. 1
17
homeostasis. IL-2R shares its β and γ subunits with the con- The missing-self hypothesis, although altered over time to
stitutively expressed IL-15R (Chapter 9). The dimeric IL-2Rβγ encompass other observations, has been extremely useful in
is able to respond to the high levels of IL-2 supplied in vitro to providing a predictive framework by which to investigate NK-cell
induce proliferation of NK cells that were historically called receptors and the recognition of target cells (Fig. 17.5).
lymphokine-activated-killer (LAK) cells. IL-2 activation will also In the past two decades, a large number of NK-cell receptors
20
induce the IL-2Rα chain to complete the high-affinity trimeric and their ligands have been identified (see Table 17.2). Although
receptor. IL-4 activates human NK cells and promotes the a number of these NK-cell receptor ligands are MHC class I