Page 267 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 267
248 PART TwO Host Defense Mechanisms and Inflammation
Immunological
synapse
CTL Target cell
LFA-1
ICAM-1
Nucleus
MTOC
Caspases
TCR MHC-I
FAS
FasL Mitochondrion
Perforin LFA1
Granzymes
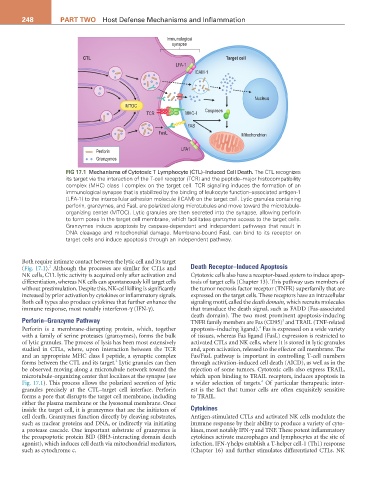
FIG 17.1 Mechanisms of Cytotoxic T Lymphocyte (CTL)–Induced Cell Death. The CTL recognizes
its target via the interaction of the T-cell receptor (TCR) and the peptide–major histocompatibility
complex (MHC) class I complex on the target cell. TCR signaling induces the formation of an
immunological synapse that is stabilized by the binding of leukocyte function–associated antigen-1
(LFA-1) to the intercellular adhesion molecule (ICAM) on the target cell. Lytic granules containing
perforin, granzymes, and FasL are polarized along microtubules and move toward the microtubule-
organizing center (MTOC). Lytic granules are then secreted into the synapse, allowing perforin
to form pores in the target cell membrane, which facilitates granzyme access to the target cells.
Granzymes induce apoptosis by caspase-dependent and independent pathways that result in
DNA cleavage and mitochondrial damage. Membrane-bound FasL can bind to its receptor on
target cells and induce apoptosis through an independent pathway.
Both require intimate contact between the lytic cell and its target
2
(Fig. 17.1). Although the processes are similar for CTLs and Death Receptor–Induced Apoptosis
NK cells, CTL lytic activity is acquired only after activation and Cytotoxic cells also have a receptor-based system to induce apop-
differentiation, whereas NK cells can spontaneously kill target cells tosis of target cells (Chapter 13). This pathway uses members of
without prestimulation. Despite this, NK-cell killing is significantly the tumor necrosis factor receptor (TNFR) superfamily that are
increased by prior activation by cytokines or inflammatory signals. expressed on the target cells. These receptors have an intracellular
Both cell types also produce cytokines that further enhance the signaling motif, called the death domain, which recruits molecules
immune response, most notably interferon-γ (IFN-γ). that transduce the death signal, such as FADD (Fas-associated
death domain). The two most prominent apoptosis-inducing
Perforin–Granzyme Pathway TNFR family members are Fas (CD95) and TRAIL (TNF-related
3
4
Perforin is a membrane-disrupting protein, which, together apoptosis–inducing ligand). Fas is expressed on a wide variety
with a family of serine proteases (granzymes), forms the bulk of tissues, whereas Fas ligand (FasL) expression is restricted to
of lytic granules. The process of lysis has been most extensively activated CTLs and NK cells, where it is stored in lytic granules
studied in CTLs, where, upon interaction between the TCR and, upon activation, released to the effector cell membrane. The
and an appropriate MHC class I peptide, a synaptic complex Fas/FasL pathway is important in controlling T-cell numbers
2
forms between the CTL and its target. Lytic granules can then through activation-induced cell death (AICD), as well as in the
be observed moving along a microtubule network toward the rejection of some tumors. Cytotoxic cells also express TRAIL,
microtubule-organizing center that localizes at the synapse (see which upon binding to TRAIL receptors, induces apoptosis in
4
Fig. 17.1). This process allows the polarized secretion of lytic a wider selection of targets. Of particular therapeutic inter-
granules precisely at the CTL–target cell interface. Perforin est is the fact that tumor cells are often exquisitely sensitive
forms a pore that disrupts the target cell membrane, including to TRAIL.
either the plasma membrane or the lysosomal membrane. Once
inside the target cell, it is granzymes that are the initiators of Cytokines
cell death. Granzymes function directly by cleaving substrates, Antigen-stimulated CTLs and activated NK cells modulate the
such as nuclear proteins and DNA, or indirectly via initiating immune response by their ability to produce a variety of cyto-
a protease cascade. One important substrate of granzymes is kines, most notably IFN-γ and TNF. These potent inflammatory
the proapoptotic protein BID (BH3-interacting domain death cytokines activate macrophages and lymphocytes at the site of
agonist), which induces cell death via mitochondrial mediators, infection. IFN-γ helps establish a T-helper cell-1 (Th1) response
such as cytochrome c. (Chapter 16) and further stimulates differentiated CTLs. NK