Page 343 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 343
CHaPtEr 22 Phagocyte Deficiencies 323
Leukocyte Adhesion Defect-2
A distinct defect of leukocyte adhesion with susceptibility to
infection was described by Etzioni et al. in 1992 and named
LAD-2. 15,19 It is characterized by growth retardation and mental
retardation, hypotonia, seizures, dysmorphic features, strabismus,
and persistent periodontitis. In contrast to LAD-1, wound healing
is not impaired, nor is the susceptibility to bacterial infections
X
as severe. Hypofucosylation of the protein CD15s (sialyl Lewis ,
X
SLe ) on neutrophils impairs the rolling step of neutrophil
adhesion. The underlying defect is in guanosine diphosphate
(GDP)–fucose biosynthesis, resulting from mutations in the
GDP–fucose transporter-1 (FUCT1 or SLC35C1), hence the
designation of this disease as a congenital disorder of glycosylation
IIc (CDGIIc). In addition to severe impairment in neutrophil
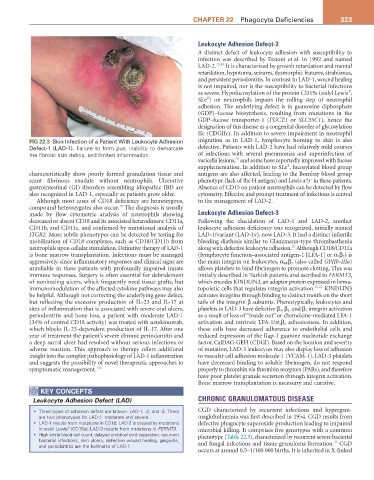
FIG 22.3 Skin Infection of a Patient With Leukocyte Adhesion migration as in LAD-1, lymphocyte homing to skin is also
Defect-1 (LAD-1). Failure to form pus, inability to demarcate defective. Patients with LAD-2 have had relatively mild courses
the fibrotic skin debris, and limited inflammation. of infections with several pneumonias and superinfection of
19
varicella lesions, and some have reportedly improved with fucose
X
supplementation. In addition to SLe , fucosylated blood group
characteristically show poorly formed granulation tissue and antigens are also affected, leading to the Bombay blood group
− −
scant fibrinous exudate without neutrophils. Ulcerative phenotype (lack of the H antigen) and Lewis a b in these patients.
gastrointestinal (GI) disorders resembling idiopathic IBD are Absence of CD15 on patient neutrophils can be detected by flow
also recognized in LAD-1, especially as patients grow older. cytometry. Effective and prompt treatment of infections is central
Although most cases of CD18 deficiency are homozygous, to the management of LAD-2.
18
compound heterozygotes also occur. The diagnosis is usually
made by flow cytometric analysis of neutrophils showing Leukocyte Adhesion Defect-3
decreased or absent CD18 and its associated heterodimers: CD11a, Following the elucidation of LAD-1 and LAD-2, another
CD11b, and CD11c, and confirmed by mutational analysis of leukocyte adhesion deficiency was recognized, initially named
ITGB2. More subtle phenotypes can be detected by testing for LAD-1/variant (LAD-1v), now LAD-3. It had a distinct infantile
mobilization of CD18 complexes, such as CD18/CD11b from bleeding diathesis similar to Glanzmann-type thrombasthenia
20
neutrophils upon cellular stimulation. Definitive therapy of LAD-1 along with defective leukocyte adhesion. Although CD18/CD11a
is bone marrow transplantation. Infections must be managed (lymphocyte function–associated antigen-1 [LFA-1] or α 1 β 2 ) is
aggressively, since inflammatory responses and clinical signs are the main integrin on leukocytes, α IIb β 3 (also called GPIIb-IIIa)
unreliable in these patients with profoundly impaired innate allows platelets to bind fibrinogen to promote clotting. This was
immune responses. Surgery is often essential for debridement initially described in Turkish patients and ascribed to FERMT3,
of nonhealing ulcers, which frequently need tissue grafts, but which encodes KINDLIN3, an adaptor protein expressed in hema-
immunomodulation of the affected cytokine pathways may also topoietic cells that regulates integrin activation. 21,22 KINDLIN3
be helpful. Although not correcting the underlying gene defect, activates integrins through binding to distinct motifs on the short
but reflecting the excessive production of IL-23 and IL-17 at tails of the integrin β subunits. Phenotypically, leukocytes and
sites of inflammation that is associated with severe oral ulcers, platelets in LAD-3 have defective β 3 , β 2 , and β 1 integrin activation
periodontitis and bone loss, a patient with moderate LAD-1 as a result of loss of “inside out” or chemokine-mediated LFA-1
(34% of control CD18 activity) was treated with ustekinumab, activation and intrinsic LFA-1/α 1 β 2 adhesiveness. In addition,
which blocks IL-23-dependent production of IL-17. After one these cells have decreased adherence to endothelial cells and
year of treatment the patient’s severe chronic periodontitis and reduced expression of the Rap-1 guanine nucleotide exchange
a deep sacral ulcer had resolved without serious infections or factor, CalDAG-GEFI (CDGI). Based on the location and severity
adverse reaction. This approach to therapy offers additional of mutation, LAD-3 leukocytes may also display loss of adhesion
insight into the complex pathophysiology of LAD-1 inflammation to vascular cell adhesion molecule-1 (VCAM-1). LAD-3 platelets
and suggests the possibility of novel therapeutic approaches to have decreased binding to soluble fibrinogen, do not respond
symptomatic management. 18a properly to thrombin via thrombin receptors (PARs), and therefore
have poor platelet granule secretion through integrin activation.
Bone marrow transplantation is necessary and curative.
KEY CoNCEPtS
Leukocyte Adhesion Defect (LAD) CHRONIC GRANULOMATOUS DISEASE
• Three types of adhesion defect are known: LAD-1, -2, and -3. There CGD characterized by recurrent infections and hypergam-
are two phenotypes for LAD-1: moderate and severe. maglobulinemia was first described in 1954. CGD results from
• LAD-1 results from mutations in CD18; LAD-2 is caused by mutations defective phagocyte superoxide production leading to impaired
in sialyl Lewis (CD15s); LAD-3 results from mutations in FERMT3. microbial killing. It comprises five genotypes with a common
X
• High white blood cell count, delayed umbilical cord separation, recurrent phenotype (Table 22.3), characterized by recurrent severe bacterial
bacterial infections, skin ulcers, defective wound healing, gingivitis, 23
and periodontitis are the hallmarks of LAD-1. and fungal infections and tissue granuloma formation. CGD
occurs at around 0.5–1/100 000 births. It is inherited in X-linked