Page 384 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 384
366 Part tHrEE Host Defenses to Infectious Agents
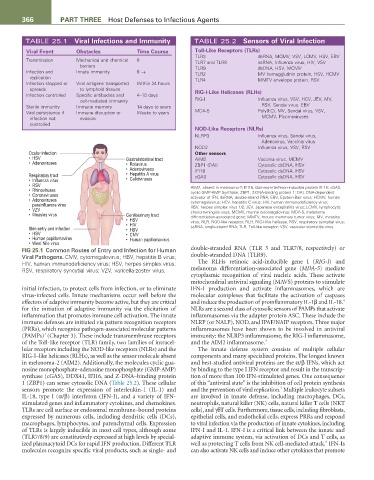
TABLE 25.1 Viral Infections and Immunity TABLE 25.2 Sensors of Viral Infection
Viral Event Obstacles time Course toll-Like receptors (tLrs)
TLR3 dsRNA, MCMV, VSV, LCMV, HSV, EBV
Transmission Mechanical and chemical 0 TLR7 and TLR8 ssRNA, Influenza virus, HIV, VSV
barriers TLR9 dsDNA, HSV, MCMV
Infection and Innate immunity 0 → TLR2 MV hemagglutinin protein, HSV, HCMV
replication TLR4 MMTV envelope protein, RSV
Infection stopped or Viral antigens transported Within 24 hours
spreads to lymphoid tissues rIG-I-Like Helicases (rLHs)
Infection controlled Specific antibodies and 4–10 days
cell-mediated immunity RIG-I Influenza virus, VSV, HCV, JEV, MV,
Sterile immunity Immune memory 14 days to years RSV, Sendai virus, EBV
Viral persistence if Immune disruption or Weeks to years MDA-5 Poly(I:C), MV, Sendai virus, VSV,
infection not evasion MCMV, Picornaviruses
controlled
NOD-Like receptors (NLrs)
NLRP3 Influenza virus, Sendai virus,
Adenovirus, Vaccinia virus
NOD2 Influenza virus, VSV, RSV
Ocular infection Other sensors
• HSV Gastrointestinal tract AIM2 Vaccinia virus, MCMV
• Adenoviruses • Rotavirus ZBP1 (DAI) Cytosolic dsDNA, HSV
• Adenoviruses IFI16 Cytosolic dsDNA, HSV
Respiratory tract • Hepatitis A virus cGAS Cytosolic dsDNA, HSV
• Influenza virus • Caliciviruses
• RSV AIM2, absent in melanoma-2; IFI16, Gamma-interferon-inducible protein Ifi-16; cGAS,
• Rhinoviruses cyclic GMP-AMP Synthase; ZBP1, Z-DNA-binding protein 1; DAI, DNA-dependent
• Coronaviruses activator of IFN; dsRNA, double-strand RNA; EBV, Epstein-Barr virus; HCMV, human
• Adenoviruses cytomegalovirus; HCV, hepatitis C virus; HIV, human immunodeficiency virus;
parainfluenza virus HSV, herpes simplex virus 1/2; JEV, Japanese encephalitis virus; LCMV, lymphocytic
• VZV choriomeningitis virus; MCMV, murine cytomegalovirus; MDA-5, melanoma
• Measles virus Genitourinary tract differentiation-associated gene; MMTV, mouse mammary tumor virus; MV, measles
• HSV virus; NLR, NOD-like receptor; RLH, RIG-I-like helicase; RSV, respiratory syncytial virus;
• HIV
Skin entry and infection • HBV ssRNA, single-strand RNA; TLR, Toll-like receptor; VSV, vesicular stomatitis virus.
• HSV • CMV
• Human papillomavirus • Human papillomavirus
• West Nile virus
FIG 25.1 Common Routes of Entry and Infection for Human double-stranded RNA (TLR 3 and TLR7/8, respectively) or
Viral Pathogens. CMV, cytomegalovirus; HBV, hepatitis B virus; double-stranded DNA (TLR9).
HIV, human immunodeficiency virus; HSV, herpes simplex virus; The RLHs retinoic acid-inducible gene I (RIG-I) and
RSV, respiratory syncytial virus; VZV, varicella-zoster virus. melanoma differentiation-associated gene (MDA-5) mediate
cytoplasmic recognition of viral nucleic acids. These activate
mitochondrial antiviral signaling (MAVS) proteins to stimulate
initial infection, to protect cells from infection, or to eliminate IFN-I production and activate inflammasomes, which are
virus-infected cells. Innate mechanisms occur well before the molecular complexes that facilitate the activation of caspases
4
effectors of adaptive immunity become active, but they are critical and induce the production of proinflammatory IL-1β and IL-18.
for the initiation of adaptive immunity via the elicitation of NLRs are a second class of cytosolic sensors of PAMPs that activate
inflammation that promotes immune cell activation. The innate inflammasomes via the adapter protein ASC. These include the
immune defenses are initiated via pattern recognition receptors NLRP (or NALP), NOD, and IPAF/NAIP receptors. Three major
(PRRs), which recognize pathogen-associated molecular patterns inflammasomes have been shown to be involved in antiviral
3
(PAMPs) (Chapter 3). These include transmembrane receptors immunity: the NLRP3 inflammasome, the RIG-I inflammasome,
of the Toll-like receptor (TLR) family, two families of intracel- and the AIM2 inflammasome. 3
lular receptors including the NOD-like receptors (NLRs) and the The innate defense system consists of multiple cellular
RIG-I–like helicases (RLHs), as well as the sensor molecule absent components and many specialized proteins. The longest known
in melanoma-2 (AIM2). Additionally, the molecules cyclic gua- and best-studied antiviral proteins are the α/β IFNs, which act
nosine monophosphate–adenosine monophosphate (GMP-AMP) by binding to the type I IFN receptor and result in the transcrip-
synthase (cGAS), DDX41, IFI16, and Z-DNA–binding protein tion of more than 100 IFN-stimulated genes. One consequence
1 (ZBP1) can sense cytosolic DNA (Table 25.2). These cellular of this “antiviral state” is the inhibition of cell protein synthesis
5
sensors promote the expression of interleukin-1 (IL-1) and and the prevention of viral replication. Multiple leukocyte subsets
IL-18, type I (α/β) interferon (IFN-I), and a variety of IFN- are involved in innate defense, including macrophages, DCs,
stimulated genes and inflammatory cytokines, and chemokines. neutrophils, natural killer (NK) cells, natural killer T cells (NKT
TLRs are cell surface or endosomal membrane–bound proteins cells), and γδT cells. Furthermore, tissue cells, including fibroblasts,
expressed by numerous cells, including dendritic cells (DCs), epithelial cells, and endothelial cells, express PRRs and respond
macrophages, lymphocytes, and parenchymal cells. Expression to viral infection via the production of innate cytokines, including
of TLRs is largely inducible in most cell types, although some IFN-I and IL-1. IFN-I is a critical link between the innate and
(TLR7/8/9) are constitutively expressed at high levels by special- adaptive immune system, via activation of DCs and T cells, as
6
ized plasmacytoid DCs for rapid IFN production. Different TLR well as protecting T cells from NK cell-mediated attack. IFN-Is
molecules recognize specific viral products, such as single- and can also activate NK cells and induce other cytokines that promote