Page 399 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 399
380 PARt tHREE Host Defenses to Infectious Agents
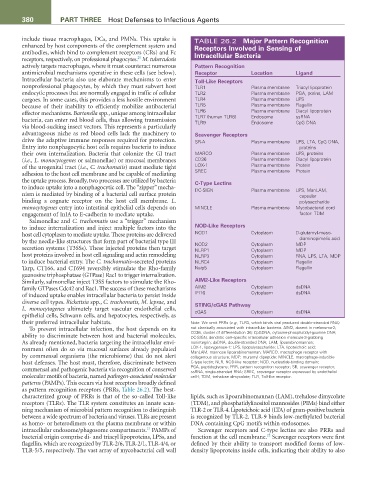
include tissue macrophages, DCs, and PMNs. This uptake is TABLE 26.2 Major Pattern Recognition
enhanced by host components of the complement system and Receptors Involved in Sensing of
antibodies, which bind to complement receptors (CRs) and Fc Intracellular Bacteria
20
receptors, respectively, on professional phagocytes. M. tuberculosis
actively targets macrophages, where it must counteract numerous Pattern Recognition
antimicrobial mechanisms operative in these cells (see below). Receptor Location Ligand
Intracellular bacteria also use elaborate mechanisms to enter toll-Like Receptors
nonprofessional phagocytes, by which they must subvert host TLR1 Plasma membrane Triacyl lipoprotein
endocytic processes that are normally engaged in traffic of cellular TLR2 Plasma membrane PGA, porins, LAM
cargoes. In some cases, this provides a less hostile environment TLR4 Plasma membrane LPS
because of their inability to efficiently mobilize antibacterial TLR5 Plasma membrane Flagellin
effector mechanisms. Bartonella spp., unique among intracellular TLR6 Plasma membrane Diacyl lipoprotein
bacteria, can enter red blood cells, thus allowing transmission TLR7 (human TLR8) Endosome ssRNA
CpG DNA
Endosome
TLR9
via blood-sucking insect vectors. This represents a particularly
advantageous niche as red blood cells lack the machinery to Scavenger Receptors
drive the adaptive immune responses required for protection. SR-A Plasma membrane LPS, LTA, CpG DNA,
Entry into nonphagocytic host cells requires bacteria to induce proteins
their own internalization. Bacteria that colonize the GI tract MARCO Plasma membrane LPS, proteins
(i.e., L. monocytogenes or salmonellae) or mucosal membranes CD36 Plasma membrane Diacyl lipoprotein
of the urogenital tract (i.e., C. trachomatis) must mediate tight LOX-1 Plasma membrane Protein
adhesion to the host cell membrane and be capable of mediating SREC Plasma membrane Protein
the uptake process. Broadly, two processes are utilized by bacteria C-type Lectins
to induce uptake into a nonphagocytic cell. The “zipper” mecha- DC-SIGN Plasma membrane LPS, ManLAM,
nism is mediated by binding of a bacterial cell surface protein capsular
binding a cognate receptor on the host cell membrane. L. polysaccharide
monocytogenes entry into intestinal epithelial cells depends on MINCLE Plasma membrane Mycobacterial cord
engagement of InIA to E-cadherin to mediate uptake. factor: TDM
Salmonellae and C. trachomatis use a “trigger” mechanism
to induce internalization and inject multiple factors into the NOD-Like Receptors
host cell cytoplasm to mediate uptake. These proteins are delivered NOD1 Cytoplasm D-glutamyl-meso-
diaminopimelic acid
by the needle-like structures that form part of bacterial type III NOD2 Cytoplasm MDP
secretion systems (T3SSs). These injected proteins then target NLRP1 Cytoplasm MDP
host proteins involved in host cell signaling and actin remodeling NLRP3 Cytoplasm RNA, LPS, LTA, MDP
to induce bacterial entry. The C. trachomatis–secreted proteins NLRC4 Cytoplasm Flagellin
Tarp, CT166, and CT694 reversibly stimulate the Rho-family Naip5 Cytoplasm Flagellin
guanosine triphosphatase (GTPase) Rac1 to trigger internalization.
Similarly, salmonellae inject T3SS factors to stimulate the Rho- AIM2-Like Receptors
family GTPases Cdc42 and Rac1. The success of these mechanisms AIM2 Cytoplasm dsDNA
of induced uptake enables intracellular bacteria to persist inside IFI16 Cytoplasm dsDNA
diverse cell types. Rickettsia spp., C. trachomatis, M. leprae, and StING/cGAS Pathway
L. monocytogenes ultimately target vascular endothelial cells, cGAS Cytoplasm dsDNA
epithelial cells, Schwann cells, and hepatocytes, respectively, as
their preferred intracellular habitats. Note: We omit PRRs (e.g., TLR3, which binds viral produced double-stranded RNA)
To prevent intracellular infection, the host depends on its not classically associated with intracellular bacteria. AIM2, absent in melanoma-2;
ability to discriminate between host and bacterial molecules. CD36, cluster of differentiation 36; CpGDNA, cytosine-phosphatidyl-guanine DNA;
DC-SIGN, dendritic cell–specific intercellular adhesion molecule-3-grabbing
As already mentioned, bacteria targeting the intracellular envi- nonintegrin; dsDNA, double-stranded DNA; LAM, lipoarabinomannan;
ronment often do so via mucosal surfaces already populated LOX-1, lipoxygenase-1; LPS, lipopolysaccharide; LTA, lipoteichoic acid;
ManLAM, mannose lipoarabinomannan; MARCO, macrophage receptor with
by commensal organisms (the microbiome) that do not alert collagenous structure; MDP, muramyl dipeptide; MINCLE, macrophage-inducible
host defenses. The host must, therefore, discriminate between C-type lectin; NLR, NOD-like receptor; NOD, nucleotide-binding domain;
commensal and pathogenic bacteria via recognition of conserved PGA, peptidoglycans; PRR, pattern recognition receptor; SR, scavenger receptor;
ssRNA, single-stranded RNA; SREC, scavenger receptor expressed by endothelial
molecular motifs of bacteria, named pathogen-associated molecular cell-I; TDM, trehalose dimycolate; TLR, Toll-like receptor.
patterns (PAMPs). This occurs via host receptors broadly defined
as pattern recognition receptors (PRRs, Table 26.2). The best-
characterized group of PRRs is that of the so-called Toll-like lipids, such as lipoarabinomannan (LAM), trehalose dimycolate
receptors (TLRs). The TLR system constitutes an innate scan- (TDM), and phosphatidylinositol mannosides (PIMs) bind either
ning mechanism of microbial pattern recognition to distinguish TLR-2 or TLR-4. Lipoteichoic acid (LTA) of gram-positive bacteria
between a wide spectrum of bacteria and viruses. TLRs are present is recognized by TLR-2. TLR-9 binds low-methylated bacterial
as homo- or heterodimers on the plasma membrane or within DNA containing CpG motifs within endosomes.
21
intracellular endosome/phagosome compartments. PAMPs of Scavenger receptors and C-type lectins are also PRRs and
22
bacterial origin comprise di- and triacyl lipoproteins, LPSs, and function at the cell membrane. Scavenger receptors were first
flagellin, which are recognized by TLR-2/6, TLR-2/1, TLR-4/4, or defined by their ability to transport modified forms of low-
TLR-5/5, respectively. The vast array of mycobacterial cell wall density lipoproteins inside cells, indicating their ability to also