Page 475 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 475
456 ParT FOur Immunological Deficiencies
10 3 10 3
A1 A2 A1 A2
10 2 10 2
CD4 PE 10 1 CD4 PE 10 1
10 0 A3 A4 10 0 A3 A4
10 0 10 1 10 2 10 3 10 0 10 1 10 2 10 3
A CD3 FITC B CD3 FITC
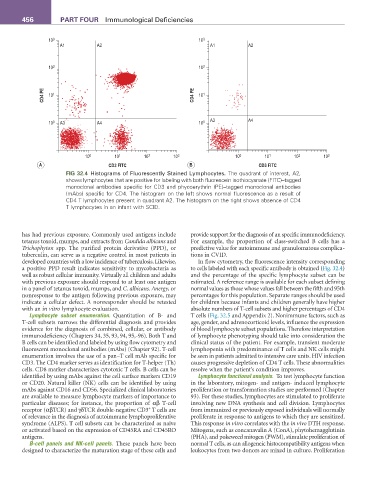
FIG 32.4 Histograms of Fluorescently Stained Lymphocytes. The quadrant of interest, A2,
shows lymphocytes that are positive for labeling with both fluorescein isothiocyanate (FITC)–tagged
monoclonal antibodies specific for CD3 and phycoerythrin (PE)–tagged monoclonal antibodies
(mAbs) specific for CD4. The histogram on the left shows normal fluorescence as a result of
CD4 T lymphocytes present in quadrant A2. The histogram on the right shows absence of CD4
T lymphocytes in an infant with SCID.
has had previous exposure. Commonly used antigens include provide support for the diagnosis of an specific immunodeficiency.
tetanus toxoid, mumps, and extracts from Candida albicans and For example, the proportion of class-switched B cells has a
Trichophyton spp. The purified protein derivative (PPD), or predictive value for autoimmune and granulomatous complica-
tuberculin, can serve as a negative control in most patients in tions in CVID.
developed countries with a low incidence of tuberculosis. Likewise, In flow cytometry, the fluorescence intensity corresponding
a positive PPD result indicates sensitivity to mycobacteria as to cells labeled with each specific antibody is obtained (Fig. 32.4)
well as robust cellular immunity. Virtually all children and adults and the percentage of the specific lymphocyte subset can be
with previous exposure should respond to at least one antigen estimated. A reference range is available for each subset defining
in a panel of tetanus toxoid, mumps, and C. albicans. Anergy, or normal values as those whose values fall between the fifth and 95th
nonresponse to the antigen following previous exposure, may percentages for this population. Separate ranges should be used
indicate a cellular defect. A nonresponder should be retested for children because infants and children generally have higher
with an in vitro lymphocyte evaluation. absolute numbers of T-cell subsets and higher percentages of CD4
Lymphocyte subset enumeration. Quantitation of B- and T cells (Fig. 32.5 and Appendix 2). Nonimmune factors, such as
T-cell subsets narrows the differential diagnosis and provides age, gender, and adrenocorticoid levels, influence the expression
evidence for the diagnosis of combined, cellular, or antibody of blood lymphocyte subset populations. Therefore interpretation
immunodeficiency (Chapters 34, 35, 93, 94, 95,-96). Both T and of lymphocyte phenotyping should take into consideration the
B cells can be identified and labeled by using flow cytometry and clinical status of the patient. For example, transient moderate
fluorescent monoclonal antibodies (mAbs) (Chapter 92). T-cell lymphopenia with predominance of T cells and NK cells might
enumeration involves the use of a pan–T cell mAb specific for be seen in patients admitted to intensive care units. HIV infection
CD3. The CD4 marker serves as identification for T-helper (Th) causes progressive depletion of CD4 T cells. These abnormalities
cells. CD8 marker characterizes cytotoxic T cells. B cells can be resolve when the patient’s condition improves.
identified by using mAbs against the cell surface markers CD19 Lymphocyte functional analysis. To test lymphocyte function
or CD20. Natural killer (NK) cells can be identified by using in the laboratory, mitogen- and antigen- induced lymphocyte
mAbs against CD16 and CD56. Specialized clinical laboratories proliferation or transformation studies are performed (Chapter
are available to measure lymphocyte markers of importance to 93). For these studies, lymphocytes are stimulated to proliferate
particular diseases; for instance, the proportion of αβ T-cell involving new DNA synthesis and cell division. Lymphocytes
+
receptor (αβTCR) and γδTCR double-negative CD3 T cells are from immunized or previously exposed individuals will normally
of relevance in the diagnosis of autoimmune lymphoproliferative proliferate in response to antigens to which they are sensitized.
syndrome (ALPS). T cell subsets can be characterized as naïve This response in vitro correlates with the in vivo DTH response.
or activated based on the expression of CD45RA and CD45RO Mitogens, such as concanavalin A (ConA), phytohemagglutinin
antigens. (PHA), and pokeweed mitogen (PWM), stimulate proliferation of
B-cell panels and NK-cell panels. These panels have been normal T cells, as can allogeneic histocompatibility antigens when
designed to characterize the maturation stage of these cells and leukocytes from two donors are mixed in culture. Proliferation