Page 575 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 575
552 ParT fOur Immunological Deficiencies
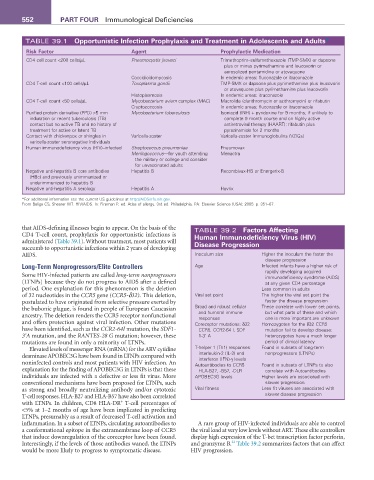
TABLE 39.1 Opportunistic Infection Prophylaxis and Treatment in adolescents and adults*
risk factor agent Prophylactic Medication
CD4 cell count <200 cells/µL Pneumocystis jiroveci Trimethoprim–sulfamethoxazole (TMP-SMX) or dapsone
plus or minus pyrimethamine and leucovorin or
aerosolized pentamidine or atovaquone
Coccidioidomycosis In endemic areas: fluconazole or itraconazole
CD4 T-cell count <100 cells/µL Toxoplasma gondii TMP-SMX or dapsone plus pyrimethamine plus leucovorin
or atovaquone plus pyrimethamine plus leucovorin
Histoplasmosis In endemic areas: itraconazole
CD4 T-cell count <50 cells/µL Mycobacterium avium complex (MAC) Macrolide (clarithromycin or azithromycin) or rifabutin
Cryptococcosis In endemic areas: fluconazole or itraconazole
Purified protein derivative (PPD) >5 mm Mycobacterium tuberculosis Isoniazid (INH) + pyridoxine for 9 months; if unlikely to
induration or recent tuberculosis (TB) complete 9 month course and on highly active
contact but no active TB and no history of antiretroviral therapy (HAART): rifabutin plus
treatment for active or latent TB pyrazinamide for 2 months
Contact with chickenpox or shingles in Varicella-zoster Varicella-zoster immunoglobulins (VZIGs)
varicella-zoster seronegative individuals
Human immunodeficiency virus (HIV)–infected Streptococcus pneumoniae Pneumovax
Meningococcus—for youth attending Menactra
the military or college and consider
for unvaccinated adults
Negative anti-hepatitis B core antibodies Hepatitis B Recombivax-HB or Energerix-B
(HBc) and previously unimmunized or
underimmunized to hepatitis B
Negative anti-hepatitis A serology Hepatitis A Havrix
*For additional information see the current US guidelines at http://AIDSinfo.nih.gov.
From Baliga CS, Shearer WT. HIV/AIDS. In: Fireman P, ed. Atlas of allergy. 3rd ed. Philadelphia, PA: Elsevier Science (USA); 2005: p. 351–67.
that AIDS-defining illnesses begin to appear. On the basis of the TABLE 39.2 factors affecting
CD4 T-cell count, prophylaxis for opportunistic infections is Human Immunodeficiency Virus (HIV)
administered (Table 39.1). Without treatment, most patients will Disease Progression
succumb to opportunistic infections within 2 years of developing
AIDS. Inoculum size Higher the inoculum the faster the
disease progression
Long-Term Nonprogressors/Elite Controllers Age Infected infants have a higher risk of
Some HIV-infected patients are called long-term nonprogressors rapidly developing acquired
immunodeficiency syndrome (AIDS)
(LTNPs) because they do not progress to AIDS after a defined at any given CD4 percentage
period. One explanation for this phenomenon is the deletion Less common in adults
of 32 nucleotides in the CCR5 gene (CCR5-δ32). This deletion, Viral set point The higher the viral set point the
postulated to have originated from selective pressure exerted by faster the disease progression
the bubonic plague, is found in people of European Caucasian Broad and robust cellular These correlate with lower set points,
ancestry. The deletion renders the CCR5 receptor nonfunctional and humoral immune but what parts of these and which
responses
one is more important are unknown
and offers protection against viral infection. Other mutations Coreceptor mutations: δ32 Homozygotes for the δ32 CCR5
have been identified, such as the CCR2-64I mutation, the SDF1- CCR5, CCR2-64 l, SDF mutation fail to develop disease;
3’A mutation, and the RANTES-28 G mutation; however, these 1-3’ A heterozygotes have a much longer
mutations are found in only a minority of LTNPs. period of clinical latency
Elevated levels of messenger RNA (mRNA) for the ARV cytidine T-helper 1 (Th1) responses: Found in subsets of long-term
deaminase APOBEC3G have been found in LTNPs compared with interleukin-2 (IL-2) and nonprogressors (LTNPs)
interferon (IFN)-γ levels
noninfected controls and most patients with HIV infection. An Autoantibodies to CCR5 Found in subsets of LTNPs to also
explanation for the finding of APOBEC3G in LTNPs is that these HLA-B27, -B57, -DLR correlate with Autoantibodies
individuals are infected with a defective or less fit virus. More APOBEC3G levels Higher levels are associated with
conventional mechanisms have been proposed for LTNPs, such slower progression
as strong and broadly neutralizing antibody and/or cytotoxic Viral fitness Less fit viruses are associated with
T-cell responses. HLA-B27 and HLA-B57 have also been correlated slower disease progression
+
with LTNPs. In children, CD8 HLA-DR T-cell percentages of
<5% at 1–2 months of age have been implicated in predicting
LTNPs, presumably as a result of decreased T-cell activation and
inflammation. In a subset of LTNPs, circulating autoantibodies to A rare group of HIV-infected individuals are able to control
a conformational epitope in the extramembrane loop of CCR5 the viral load at very low levels without ART. These elite controllers
that induce downregulation of the coreceptor have been found. display high expression of the T-bet transcription factor perforin,
22
Interestingly, if the levels of those antibodies waned, the LTNPs and granzyme B. Table 39.2 summarizes factors that can affect
would be more likely to progress to symptomatic disease. HIV progression.