Page 924 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 924
892 Part Seven Organ-Specific Inflammatory Disease
that, analogous to SPMS, acute inflammatory lesions form during
early stages of PPMS, prior to overt clinical progression, but
happen to arise exclusively in clinically silent areas. Conversely,
some investigators have argued that PPMS is a distinct disease
entity driven primarily by neurodegenerative processes from its
3
inception. This viewpoint is supported by the general failure
8
of immunomodulatory agents to attenuate the course of PPMS.
Conversely, in a randomized double-blind placebo-controlled
trial, treatment with a B cell–depleting monoclonal antibody
(mAb) delayed disability progression in a subset of patients with
9
PPMS who were younger and/or had inflammatory lesions.
Collectively, these findings suggest that the pathogenesis of PPMS
is multifaceted and heterogeneous and that the relative contribu-
tion of inflammation and neurodegeneration to clinical outcomes
A B varies among patients.
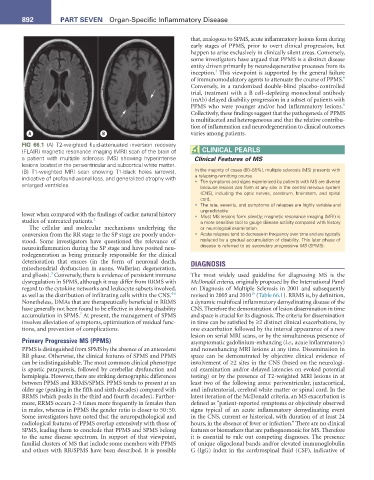
FIG 66.1 (A) T2-weighted fluid-attenuated inversion recovery
(FLAIR) magnetic resonance imaging (MRI) scan of the brain of CLInICaL PearLS
a patient with multiple sclerosis (MS) showing hyperintense Clinical Features of MS
lesions located in the periventricular and subcortical white matter.
(B) T1-weighted MRI scan showing T1-black holes (arrows), In the majority of cases (80–85%), multiple sclerosis (MS) presents with
indicative of profound axonal loss, and generalized atrophy with a relapsing-remitting course.
enlarged ventricles. • The symptoms and signs experienced by patients with MS are diverse
because lesions can form at any site in the central nervous system
(CNS), including the optic nerves, cerebrum, brainstem, and spinal
cord.
• The rate, severity, and symptoms of relapses are highly variable and
unpredictable.
lower when compared with the findings of earlier natural history • Most MS lesions form silently; magnetic resonance imaging (MRI) is
studies of untreated patients. 2 a more sensitive tool to gauge disease activity compared with history
The cellular and molecular mechanisms underlying the or neurological examination.
conversion from the RR stage to the SP stage are poorly under- • Acute relapses tend to decrease in frequency over time and are typically
stood. Some investigators have questioned the relevance of replaced by a gradual accumulation of disability. This later phase of
neuroinflammation during the SP stage and have posited neu- disease is referred to as secondary progressive MS (SPMS).
rodegeneration as being primarily responsible for the clinical
deterioration that ensues (in the form of neuronal death, DIAGNOSIS
mitochondrial dysfunction in axons, Wallerian degeneration,
3
and gliosis). Conversely, there is evidence of persistent immune The most widely used guideline for diagnosing MS is the
dysregulation in SPMS, although it may differ from RRMS with McDonald criteria, originally proposed by the International Panel
regard to the cytokine networks and leukocyte subsets involved, on Diagnosis of Multiple Sclerosis in 2001 and subsequently
4-6
10
as well as the distribution of infiltrating cells within the CNS. revised in 2005 and 2010 (Table 66.1). RRMS is, by definition,
Nonetheless, DMAs that are therapeutically beneficial in RRMS a dynamic multifocal inflammatory demyelinating disease of the
have generally not been found to be effective in slowing disability CNS. Therefore the demonstration of lesion dissemination in time
7
accumulation in SPMS. At present, the management of SPMS and space is crucial for its diagnosis. The criteria for dissemination
involves alleviation of symptoms, optimization of residual func- in time can be satisfied by ≥2 distinct clinical exacerbations, by
tions, and prevention of complications. one exacerbation followed by the interval appearance of a new
lesion on serial MRI scans, or by the simultaneous presence of
Primary Progressive MS (PPMS) asymptomatic gadolinium-enhancing (i.e., acute inflammatory)
PPMS is distinguished from SPMS by the absence of an antecedent and nonenhancing MRI lesions at any time. Dissemination in
RR phase. Otherwise, the clinical features of SPMS and PPMS space can be demonstrated by objective clinical evidence of
can be indistinguishable. The most common clinical phenotype involvement of ≥2 sites in the CNS (based on the neurologi-
is spastic paraparesis, followed by cerebellar dysfunction and cal examination and/or delayed latencies on evoked potential
hemiplegia. However, there are striking demographic differences testing) or by the presence of T2-weighted MRI lesions in at
between PPMS and RRMS/SPMS. PPMS tends to present at an least two of the following areas: periventricular, juxtacortical,
older age (peaking in the fifth and sixth decades) compared with and infratentorial, cerebral white matter or spinal cord. In the
RRMS (which peaks in the third and fourth decades). Further- latest iteration of the McDonald criteria, an MS exacerbation is
more, RRMS occurs 2–3 times more frequently in females than defined as “patient-reported symptoms or objectively observed
in males, whereas in PPMS the gender ratio is closer to 50 : 50. signs typical of an acute inflammatory demyelinating event
Some investigators have noted that the neuropathological and in the CNS, current or historical, with duration of at least 24
radiological features of PPMS overlap extensively with those of hours, in the absence of fever or infection.” There are no clinical
SPMS, leading them to conclude that PPMS and SPMS belong features or biomarkers that are pathognomonic for MS. Therefore
to the same disease spectrum. In support of that viewpoint, it is essential to rule out competing diagnoses. The presence
familial clusters of MS that include some members with PPMS of unique oligoclonal bands and/or elevated immunoglobulin
and others with RR/SPMS have been described. It is possible G (IgG) index in the cerebrospinal fluid (CSF), indicative of