Page 128 - 9780077418427.pdf
P. 128
/Users/user-f465/Desktop
tiL12214_ch04_085-114.indd Page 105 9/1/10 9:38 PM user-f465
tiL12214_ch04_085-114.indd Page 105 9/1/10 9:38 PM user-f465 /Users/user-f465/Desktop
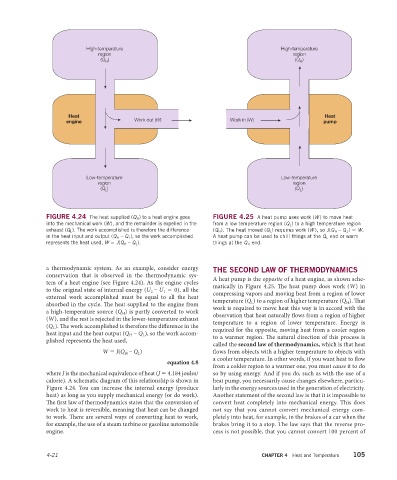
High-temperature High-temperature
region region
(Q ) (Q )
H
H
Heat Heat
Work out (W) Work in (W)
engine pump
Low-temperature Low-temperature
region region
(Q ) (Q )
L
L
FIGURE 4.24 The heat supplied (Q H ) to a heat engine goes FIGURE 4.25 A heat pump uses work (W ) to move heat
into the mechanical work (W), and the remainder is expelled in the from a low temperature region (Q L ) to a high temperature region
exhaust (Q L ). The work accomplished is therefore the difference (Q H ). The heat moved (Q L ) requires work (W ), so J(Q H – Q L ) = W.
in the heat input and output (Q H – Q L ), so the work accomplished A heat pump can be used to chill things at the Q L end or warm
represents the heat used, W = J(Q H – Q L ). things at the Q H end.
a thermodynamic system. As an example, consider energy THE SECOND LAW OF THERMODYNAMICS
conservation that is observed in the thermodynamic sys-
A heat pump is the opposite of a heat engine, as shown sche-
tem of a heat engine (see Figure 4.24). As the engine cycles
matically in Figure 4.25. The heat pump does work (W) in
to the original state of internal energy (U 2 – U 1 = 0), all the
compressing vapors and moving heat from a region of lower
external work accomplished must be equal to all the heat
temperature (Q L ) to a region of higher temperature (Q H ). Th at
absorbed in the cycle. The heat supplied to the engine from
work is required to move heat this way is in accord with the
a high-temperature source (Q H ) is partly converted to work
observation that heat naturally fl ows from a region of higher
(W), and the rest is rejected in the lower-temperature exhaust
temperature to a region of lower temperature. Energy is
(Q L ). The work accomplished is therefore the difference in the
required for the opposite, moving heat from a cooler region
heat input and the heat output (Q H – Q L ), so the work accom-
to a warmer region. The natural direction of this process is
plished represents the heat used,
called the second law of thermodynamics, which is that heat
W = J(Q H – Q L ) fl ows from objects with a higher temperature to objects with
a cooler temperature. In other words, if you want heat to fl ow
equation 4.8
from a colder region to a warmer one, you must cause it to do
where J is the mechanical equivalence of heat (J = 4.184 joules/ so by using energy. And if you do, such as with the use of a
calorie). A schematic diagram of this relationship is shown in heat pump, you necessarily cause changes elsewhere, particu-
Figure 4.24. You can increase the internal energy (produce larly in the energy sources used in the generation of electricity.
heat) as long as you supply mechanical energy (or do work). Another statement of the second law is that it is impossible to
Th e first law of thermodynamics states that the conversion of convert heat completely into mechanical energy. This does
work to heat is reversible, meaning that heat can be changed not say that you cannot convert mechanical energy com-
to work. There are several ways of converting heat to work, pletely into heat, for example, in the brakes of a car when the
for example, the use of a steam turbine or gasoline automobile brakes bring it to a stop. The law says that the reverse pro-
engine. cess is not possible, that you cannot convert 100 percent of
4-21 CHAPTER 4 Heat and Temperature 105