Page 458 - 9780077418427.pdf
P. 458
/Users/user-f465/Desktop
tiL12214_ch17_433-454.indd Page 435 9/3/10 6:20 PM user-f465
tiL12214_ch17_433-454.indd Page 435 9/3/10 6:20 PM user-f465 /Users/user-f465/Desktop
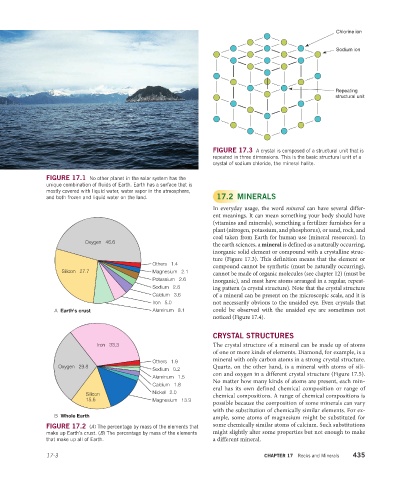
Chlorine ion
Sodium ion
Repeating
structural unit
FIGURE 17.3 A crystal is composed of a structural unit that is
repeated in three dimensions. This is the basic structural unit of a
crystal of sodium chloride, the mineral halite.
FIGURE 17.1 No other planet in the solar system has the
unique combination of fluids of Earth. Earth has a surface that is
mostly covered with liquid water, water vapor in the atmosphere,
and both frozen and liquid water on the land. 17.2 MINERALS
In everyday usage, the word mineral can have several differ-
ent meanings. It can mean something your body should have
(vitamins and minerals), something a fertilizer furnishes for a
plant (nitrogen, potassium, and phosphorus), or sand, rock, and
coal taken from Earth for human use (mineral resources). In
Oxygen 46.6
the earth sciences, a mineral is defined as a naturally occurring,
inorganic solid element or compound with a crystalline struc-
ture (Figure 17.3). This definition means that the element or
Others 1.4 compound cannot be synthetic (must be naturally occurring),
Silicon 27.7 Magnesium 2.1 cannot be made of organic molecules (see chapter 12) (must be
Potassium 2.6 inorganic), and must have atoms arranged in a regular, repeat-
Sodium 2.8 ing pattern (a crystal structure). Note that the crystal structure
Calcium 3.6 of a mineral can be present on the microscopic scale, and it is
Iron 5.0 not necessarily obvious to the unaided eye. Even crystals that
A Earth's crust Aluminum 8.1 could be observed with the unaided eye are sometimes not
noticed (Figure 17.4).
CRYSTAL STRUCTURES
Iron 33.3 The crystal structure of a mineral can be made up of atoms
of one or more kinds of elements. Diamond, for example, is a
Others 1.9 mineral with only carbon atoms in a strong crystal structure.
Oxygen 29.8 Sodium 0.2 Quartz, on the other hand, is a mineral with atoms of sili-
con and oxygen in a different crystal structure ( Figure 17.5).
Aluminum 1.5
No matter how many kinds of atoms are present, each min-
Calcium 1.8 eral has its own defined chemical composition or range of
Silicon Nickel 2.0 chemical compositions. A range of chemical compositions is
15.6 Magnesium 13.9
possible because the composition of some minerals can vary
with the substitution of chemically similar elements. For ex-
B Whole Earth ample, some atoms of magnesium might be substituted for
some chemically similar atoms of calcium. Such substitutions
FIGURE 17.2 (A) The percentage by mass of the elements that
make up Earth’s crust. (B) The percentage by mass of the elements might slightly alter some properties but not enough to make
that make up all of Earth. a different mineral.
17-3 CHAPTER 17 Rocks and Minerals 435