Page 462 - 9780077418427.pdf
P. 462
/Users/user-f465/Desktop
tiL12214_ch17_433-454.indd Page 439 9/3/10 6:20 PM user-f465
tiL12214_ch17_433-454.indd Page 439 9/3/10 6:20 PM user-f465 /Users/user-f465/Desktop
TABLE 17.2
The Mohs hardness scale
Mineral Assigned Hardness
Talc (softest) 1
Gypsum 2
Calcite 3
Fluorite 4
Apatite 5
Orthoclase 6
Quartz 7
Topaz 8
Corundum 9
Diamond (hardest) 10
Note: The hardness of some common objects is sometimes used for comparisons
rather than an actual test mineral. Here are some of the common objects and
their approximate hardnesses on the same scale: fingernail, 2.5; copper penny,
3.5; ordinary glass, 5 to 6; pocketknife blade, 5 to 6.
A
structure is cleavage, the tendency of minerals to break along
smooth planes. Where the cleavage occurs depends on zones of
weakness in the crystal structure. Mica, for example, will break
along zones of weakness into very thin sheets. Calcite and halite
will break in three directions, and if you hit either mineral with
a hammer, it will shatter into little pieces with angles consistent
with the original specimen (assuming it was cleaved).
CONCEPTS Applied
Grow Your Own
Experiment with growing crystals from solutions of alum,
copper sulfate, salt, or potassium permanganate. Write a
procedure that will tell others what the important variables
B
are for growing large, well-formed crystals.
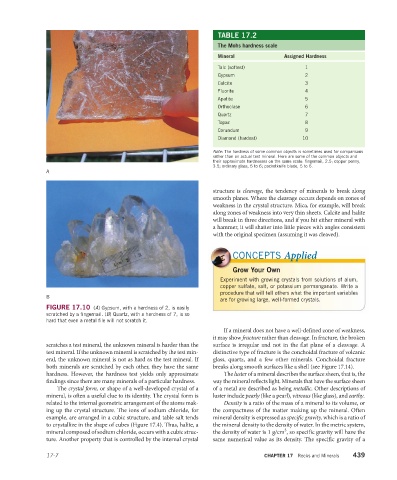
FIGURE 17.10 (A) Gypsum, with a hardness of 2, is easily
scratched by a fingernail. (B) Quartz, with a hardness of 7, is so
hard that even a metal file will not scratch it.
If a mineral does not have a well-defined zone of weakness,
it may show fracture rather than cleavage. In fracture, the broken
scratches a test mineral, the unknown mineral is harder than the surface is irregular and not in the flat plane of a cleavage. A
test mineral. If the unknown mineral is scratched by the test min- distinctive type of fracture is the conchoidal fracture of volcanic
eral, the unknown mineral is not as hard as the test mineral. If glass, quartz, and a few other minerals. Conchoidal fracture
both minerals are scratched by each other, they have the same breaks along smooth surfaces like a shell (see Figure 17.14).
hardness. However, the hardness test yields only approximate The luster of a mineral describes the surface sheen, that is, the
findings since there are many minerals of a particular hardness. way the mineral reflects light. Minerals that have the surface sheen
The crystal form, or shape of a well-developed crystal of a of a metal are described as being metallic. Other descriptions of
mineral, is often a useful clue to its identity. The crystal form is luster include pearly (like a pearl), vitreous (like glass), and earthy.
related to the internal geometric arrangement of the atoms mak- Density is a ratio of the mass of a mineral to its volume, or
ing up the crystal structure. The ions of sodium chloride, for the compactness of the matter making up the mineral. Often
example, are arranged in a cubic structure, and table salt tends mineral density is expressed as specific gravity, which is a ratio of
to crystallize in the shape of cubes (Figure 17.4). Thus, halite, a the mineral density to the density of water. In the metric system,
3
mineral composed of sodium chloride, occurs with a cubic struc- the density of water is 1 g/cm , so specific gravity will have the
ture. Another property that is controlled by the internal crystal same numerical value as its density. The specific gravity of a
17-7 CHAPTER 17 Rocks and Minerals 439