Page 169 - The City and Guilds Textbook: Plumbing Book 1 for the Level 3 Apprenticeship (9189), Level 2 Technical Certificate (8202) and Level 2 Diploma (6035)
P. 169
Chapter 3 Scientific principles
For galvanic corrosion to occur, three conditions must be present:
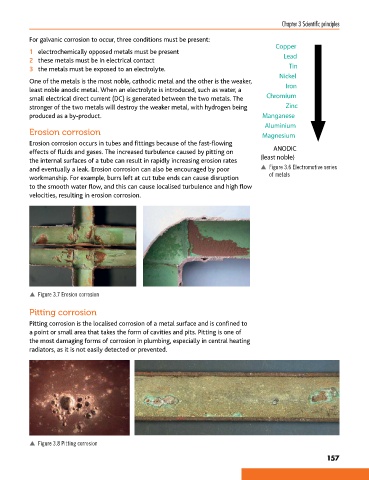
Copper
1 electrochemically opposed metals must be present Lead
2 these metals must be in electrical contact
3 the metals must be exposed to an electrolyte. Tin
Nickel
One of the metals is the most noble, cathodic metal and the other is the weaker, Iron
least noble anodic metal. When an electrolyte is introduced, such as water, a
small electrical direct current (DC) is generated between the two metals. The Chromium
stronger of the two metals will destroy the weaker metal, with hydrogen being Zinc
produced as a by-product. Manganese
Aluminium
Erosion corrosion Magnesium
Erosion corrosion occurs in tubes and fittings because of the fast-flowing
effects of fluids and gases. The increased turbulence caused by pitting on ANODIC
the internal surfaces of a tube can result in rapidly increasing erosion rates (least noble)
and eventually a leak. Erosion corrosion can also be encouraged by poor p Figure 3.6 Electromotive series
workmanship. For example, burrs left at cut tube ends can cause disruption of metals
to the smooth water flow, and this can cause localised turbulence and high flow
velocities, resulting in erosion corrosion.
p Figure 3.7 Erosion corrosion
Pitting corrosion
Pitting corrosion is the localised corrosion of a metal surface and is confined to
a point or small area that takes the form of cavities and pits. Pitting is one of
the most damaging forms of corrosion in plumbing, especially in central heating
radiators, as it is not easily detected or prevented.
p Figure 3.8 Pitting corrosion
157
9781510416482.indb 157 29/03/19 8:55 PM