Page 525 - APPLIED PROCESS DESIGN FOR CHEMICAL AND PETROCHEMICAL PLANTS, Volume 1, 3rd Edition
P. 525
Applied Process Design 491
(text continued from page 186) tion, effects of temperature and pressure are valid only
v1, v2, etc. = volume percent of each combus- for enclosed conditions, such as tanks, vessels, piping and
tionable gas present in mixture, other processing equipment. See Figures 7-49A and 7-49B
free from air and inert gas and Table 7-22 for LEL or UEL showing a variation of lim-
its with temperatures and pressures.
(7-55)
Extreme care must be exercised in designing poten-
tially flammable systems to use reliable flammability limits
L 1, � . L3 •.. = lower flammability limits, vol% for each flam- data and to recognize the effects of pressure/temperature
mable gas in mixture
on the data and its implications to the safety of the system
in question. Unless otherwise indicated, most published
Example 7-13: Calculation of LEL for Flammable Mixture
data is at atmospheric pressure and ambient temperature
and should be corrected for other conditions.
Assume mixture analysis ( combustible with air):
Methane, 3.0%, LEL = 5.3% Figure 7-4 7 illustrates a gas-freeing system using gaso-
Propane, 4.0%, LEL = 2.3% line-air-water-vapor (the water vapor could be steam). The
Hexane, LQ%, LEL = 1.1% mixture "A" represents a saturated gasoline-vapor-air-
Subtotal 8.0% combustible water-vapor mixture at 70°F. In a closed tank, a more
Balance is air (92%) volatile gasoline than the one diagrammed would give a
saturated mixture with gasoline vapor and less air. A less
For each component: v, combustible only on air-free basis. volatile gasoline would give less gasoline vapor and more
Methane.X (100) = 37.5% air. If a continuous supply of air saturated with water
Propane.X (100) = 50.0% vapor is added to a tank containing mixture A, all com-
Hexane.X (100) = 12.5% positions between A and B (air plus water vapor) will be
Percentage combustible only = 100.0% formed until all the gasoline vapor has been flushed from
the tank and only steam remains (at 212°F or higher). If
100
Mixture composite LEL = -----------=--.:..:;_ _ the tank is cooled, the steam will condense and air will be
37.::i/::i.3 + 50/2.3 + 12.5/ 1.1 drawn into the tank giving mixtures along CrB. At 70°F
only air plus a small amount of water vapor will remain.
= 2.48% volume for mixture If hot water and water vapor at l 75°F are used to flush
mixture A from the tank, the mixture composition can
The UEL for a composite is determined in the same man- only shift along AC to E. Mixtures between A and E are
ner, using the respective component UEL values. For the
overall mixture, the above can be used to calculate the flushed from the tank, mixed with air to give a mixture
between parts AE and B. After examining several other
composition. Also see Ref [52].
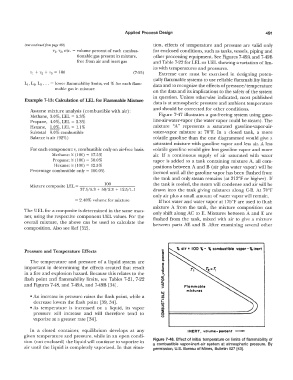
,. olr • 100 ,. _ ,. combu1tlble vopcr - ,. inert
Pressure and Temperature Effects
The temperature and pressure of a liquid system are
important in determining the effects created that result
in a fire and explosion hazard. Because this relates to the
flash point and flammability limits, see Tables 7-21, 7-22
and Figures 7-48, and 7-49A, and 7-49B [34). Flammable
mhltur11
• An increase in pressure raises the flash point, while a
decrease lowers the flash point [39, 34].
• As temperature is increased on a liquid, its vapor
pressure will increase and will therefore tend to
vaporize at a greater rate [34].
In a closed container, equilibrium develops at any INERT, volume- percent -
given temperature and pressure, while in an open condi-
tion (not enclosed) the liquid will continue to vaporize in Figure 7-48. Effect of initial temperature on limits of flammability of
a combustible vapor-inert-air system at atmospheric pressure. By
air until the liquid is completely vaporized. In that situa- permission, U.S. Bureau of Mines, Bulletin 627 [43].