Page 526 - APPLIED PROCESS DESIGN FOR CHEMICAL AND PETROCHEMICAL PLANTS, Volume 1, 3rd Edition
P. 526
492 Applied Process Design for Chemical and Petrochemical Plants
•o.ooo""
30.000
10.000
.s
19.ggg 200 ..
• �
II,
' ..
8.000 ..
1,000 100;
6,000 .
..
�000 l!> • .. •
d/)00 •
r
i 3,00!) ,0 e
:I :u
'l!>
; ·�
' 2.000
:
•
L
c.. .. �
..
'S
0
A 0
,c
�
10
"
t
'lO i
!
i
i
�
2!> I
Ttmperolllf't of liquid. O�C.
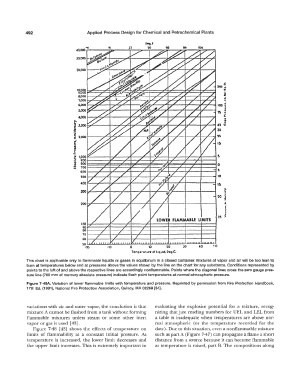
This chart is applicable only to flammable liquids or gases in equilibrium in a closed container. Mixtures of vapor and air will be too lean to
burn at temperatures below and at pressures above the values shown by the line on the chart for any substance. Conditions represented by
points to the left of and above the respective lines are accordingly nonflammable. Points where the diagonal lines cross the zero gauge pres-
sure line (760 mm of mercury absolute pressure) indicate flash point temperatures at normal atmospheric pressure.
Figure 7-49A. Variation of lower flammable limits with temperature and pressure. Reprinted by permission from Fire Protection Handbook,
17th Ed. (1991 ), National Fire Protection Association, Quincy, MA 02269 [34].
variations with air and water vapor, the conclusion is that evaluating the explosive potential for a mixture, recog-
mixture A cannot be flushed from a tank without forming nizing that just reading numbers for UEL and LEL from
flammable mixtures unless steam or some other inert a table is inadequate when temperatures are above nor-
vapor or gas is used (43]. mal atmospheric (or the temperature recorded for the
Figure 7-48 [ 43] shows the effects of temperature on date). Due to this situation, even a nonflammable mixture
limits of flammability at a constant initial pressure. As such as part A (Figure 7-47) can propagate a flame a short
temperature is increased, the lower limit decreases and distance from a source because it can become flammable
the upper limit increases. This is extremely important in as temperature is raised, part B. The compositions along