Page 41 - Spotlight A+ Form 4 & 5 Chemistry KSSM
P. 41
Form
5
Chapter 1 Redox Equilibrium Chemistry
CHAP. CHAP.
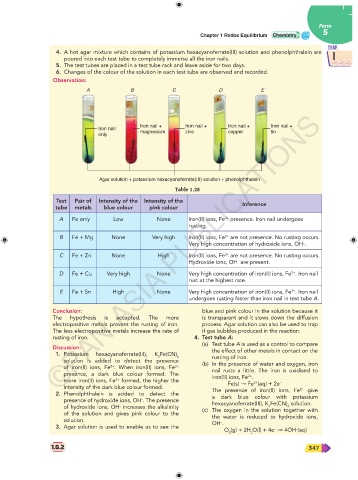
1 4. A hot agar mixture which contains of potassium hexacyanoferrate(III) solution and phenolphthalein are 1
poured into each test tube to completely immerse all the iron nails.
5. The test tubes are placed in a test tube rack and leave aside for two days.
6. Changes of the colour of the solution in each test tube are observed and recorded.
Observation:
A B C D E
©PAN ASIA PUBLICATIONS
Iron nail + Iron nail + Iron nail + Iron nail +
Iron nail
only magnesium zinc copper tin
Agar solution + potassium hexacyanoferrate(III) solution + phenolphthalein
Table 1.28
Test Pair of Intensity of the Intensity of the
tube metals blue colour pink colour Inference
A Fe only Low None Iron(II) ions, Fe presence. Iron nail undergoes
2+
rusting.
B Fe + Mg None Very high Iron(II) ions, Fe are not presence. No rusting occurs.
2+
–
Very high concentration of hydroxide ions, OH .
C Fe + Zn None High Iron(II) ions, Fe are not presence. No rusting occurs.
2+
–
Hydroxide ions, OH are present.
D Fe + Cu Very high None Very high concentration of iron(II) ions, Fe . Iron nail
2+
rust at the highest rate.
E Fe + Sn High None Very high concentration of iron(II) ions, Fe . Iron nail
2+
undergoes rusting faster than iron nail in test tube A.
Conclusion: blue and pink colour in the solution because it
The hypothesis is accepted. The more is transparent and it slows down the diffusion
electropositive metals prevent the rusting of iron. process. Agar solution can also be used to trap
The less electropositive metals increase the rate of if gas bubbles produced in the reaction.
rusting of iron. 4. Test tube A:
(a) Test tube A is used as a control to compare
Discussion: the effect of other metals in contact on the
1. Potassium hexacyanoferrate(III), K Fe(CN)
3 6 rusting of iron.
solution is added to detect the presence (b) In the presence of water and oxygen, iron
2+
of iron(II) ions, Fe . When iron(II) ions, Fe nail rusts a little. The iron is oxidised to
2+
presence, a dark blue colour formed. The 2+
2+
more iron(II) ions, Fe formed, the higher the iron(II) ions, Fe . 2+ –
Fe(s) → Fe (aq) + 2e
intensity of the dark blue colour formed. The presence of iron(II) ions, Fe give
2+
2. Phenolphthalein is added to detect the a dark blue colour with potassium
presence of hydroxide ions, OH . The presence hexacyanoferrate(III), K Fe(CN) solution.
–
–
of hydroxide ions, OH increases the alkalinity 3 6
(c) The oxygen in the solution together with
of the solution and gives pink colour to the the water is reduced to hydroxide ions,
solution. OH .
–
3. Agar solution is used to enable us to see the
–
O (g) + 2H O(l) + 4e → 4OH (aq)
–
2 2
1.6.2 347