Page 40 - Spotlight A+ Form 4 & 5 Chemistry KSSM
P. 40
Form
5 Chemistry Chapter 1 Redox Equilibrium
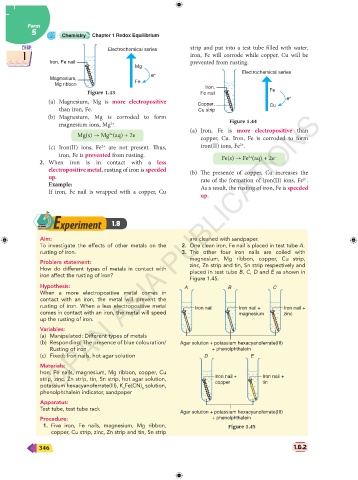
CHAP. Electrochemical series strip and put into a test tube filled with water, CHAP.
1 iron, Fe will corrode while copper, Cu will be 1
Iron, Fe nail prevented from rusting.
Mg
Electrochemical series
e –
Magnesium,
Mg ribbon Fe Iron,
Figure 1.43 Fe nail Fe
(a) Magnesium, Mg is more electropositive Copper, e –
than iron, Fe. Cu strip Cu
(b) Magnesium, Mg is corroded to form
©PAN ASIA PUBLICATIONS
magnesium ions, Mg . Figure 1.44
2+
(a) Iron, Fe is more electropositive than
Mg(s) → Mg (aq) + 2e – copper, Cu. Iron, Fe is corroded to form
2+
2+
(c) Iron(II) ions, Fe are not present. Thus, iron(II) ions, Fe .
2+
iron, Fe is prevented from rusting. Fe(s) → Fe (aq) + 2e –
2+
2. When iron is in contact with a less
electropositive metal, rusting of iron is speeded (b) The presence of copper, Cu increases the
up. rate of the formation of iron(II) ions, Fe .
2+
Example: As a result, the rusting of iron, Fe is speeded
If iron, Fe nail is wrapped with a copper, Cu
up.
1.8
Aim: are cleaned with sandpaper.
To investigate the effects of other metals on the 2. One clean iron, Fe nail is placed in test tube A.
rusting of iron. 3. The other four iron nails are coiled with
magnesium, Mg ribbon, copper, Cu strip,
Problem statement: zinc, Zn strip and tin, Sn strip respectively and
How do different types of metals in contact with
placed in test tube B, C, D and E as shown in
iron affect the rusting of iron?
Figure 1.45.
Hypothesis: A B C
When a more electropositive metal comes in
contact with an iron, the metal will prevent the
rusting of iron. When a less electropositive metal Iron nail Iron nail + Iron nail +
comes in contact with an iron, the metal will speed magnesium zinc
up the rusting of iron.
Variables:
(a) Manipulated: Different types of metals
(b) Responding: The presence of blue colouration/ Agar solution + potassium hexacyanoferrate(III)
Rusting of iron + phenolphthalein
(c) Fixed: Iron nails, hot agar solution D E
Materials:
Iron, Fe nails, magnesium, Mg ribbon, copper, Cu
strip, zinc, Zn strip, tin, Sn strip, hot agar solution, Iron nail + Iron nail +
copper
tin
potassium hexacyanoferrate(III), K Fe(CN) solution,
3 6
phenolphthalein indicator, sandpaper
Apparatus:
Test tube, test tube rack
Agar solution + potassium hexacyanoferrate(III)
Procedure: + phenolphthalein
1. Five iron, Fe nails, magnesium, Mg ribbon, Figure 1.45
copper, Cu strip, zinc, Zn strip and tin, Sn strip
346 1.6.2