Page 44 - Spotlight A+ Form 4 & 5 Chemistry KSSM
P. 44
Form
5 Chemistry Chapter 1 Redox Equilibrium
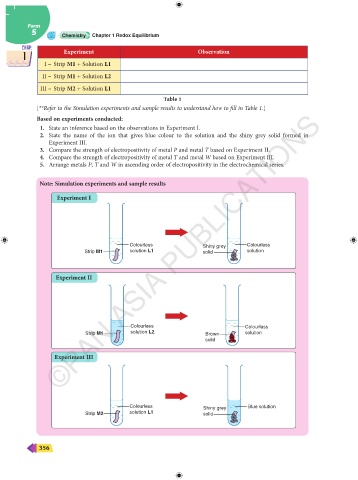
CHAP. Experiment Observation CHAP.
1 1
I – Strip M1 + Solution L1
II – Strip M1 + Solution L2
III – Strip M2 + Solution L1
Table 1
[**Refer to the Simulation experiments and sample results to understand how to fill in Table 1.]
©PAN ASIA PUBLICATIONS
Based on experiments conducted:
1. State an inference based on the observations in Experiment I.
2. State the name of the ion that gives blue colour to the solution and the shiny grey solid formed in
Experiment III.
3. Compare the strength of electropositivity of metal P and metal T based on Experiment II.
4. Compare the strength of electropositivity of metal T and metal W based on Experiment III.
5. Arrange metals P, T and W in ascending order of electropositivity in the electrochemical series.
Note: Simulation experiments and sample results
Experiment I
Colourless Shiny grey Colourless
Strip M1 solution L1 solid solution
Experiment II
Colourless Colourless
Strip M1 solution L2 Brown solution
solid
Experiment III
Colourless Shiny grey Blue solution
Strip M2 solution L1 solid
356