Page 8 - Spotlight A+ Form 4 & 5 Chemistry KSSM
P. 8
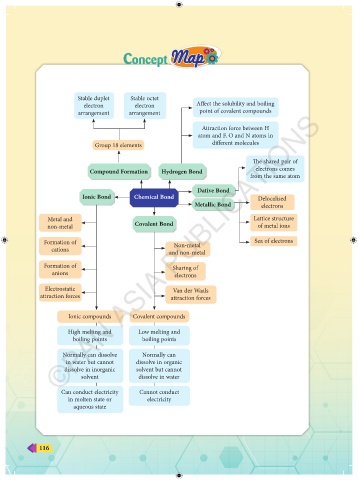
Stable duplet Stable octet
electron electron Affect the solubility and boiling
arrangement arrangement point of covalent compounds
©PAN ASIA PUBLICATIONS
Attraction force between H
atom and F, O and N atoms in
Group 18 elements different molecules
The shared pair of
Compound Formation Hydrogen Bond electrons comes
from the same atom
Dative Bond
Ionic Bond Chemical Bond Delocalised
Metallic Bond electrons
Metal and Lattice structure
non-metal Covalent Bond of metal ions
Formation of Non-metal Sea of electrons
cations and non-metal
Formation of Sharing of
anions electrons
Electrostatic Van der Waals
attraction forces attraction forces
Ionic compounds Covalent compounds
High melting and Low melting and
boiling points boiling points
Normally can dissolve Normally can
in water but cannot dissolve in organic
dissolve in inorganic solvent but cannot
solvent dissolve in water
Can conduct electricity Cannot conduct
in molten state or electricity
aqueous state
116