Page 13 - Spotlight A+ Form 4 & 5 Chemistry KSSM
P. 13
Form
4
Chapter 5 Chemical Bond Chemistry
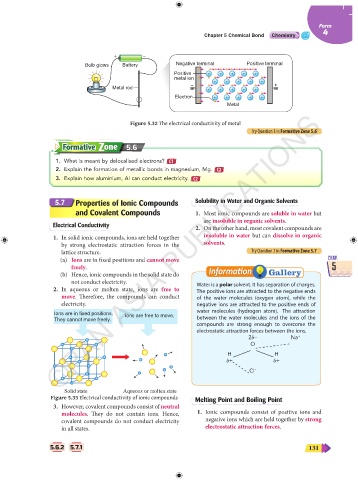
+ –
Bulb glows Battery Negative terminal Positive terminal
Positive + + + + +
metal ion
+ + + + +
– +
Metal rod + + + + +
Electron + + + + +
Metal
©PAN ASIA PUBLICATIONS
Figure 5.32 The electrical conductivity of metal
Try Question 3 in Formative Zone 5.6
5.6
1. What is meant by delocalised electrons? C1
2. Explain the formation of metallic bonds in magnesium, Mg. C2
3. Explain how aluminium, Al can conduct electricity. C2
5.7 Properties of Ionic Compounds Solubility in Water and Organic Solvents
and Covalent Compounds 1. Most ionic compounds are soluble in water but
are insoluble in organic solvents.
Electrical Conductivity 2. On the other hand, most covalent compounds are
1. In solid ionic compounds, ions are held together insoluble in water but can dissolve in organic
by strong electrostatic attraction forces in the solvents.
lattice structure. Try Question 2 in Formative Zone 5.7
CHAP. (a) Ions are in fixed positions and cannot move CHAP.
5 freely. 5
(b) Hence, ionic compounds in the solid state do
not conduct electricity. Water is a polar solvent. It has separation of charges.
2. In aqueous or molten state, ions are free to The positive ions are attracted to the negative ends
move. Therefore, the compounds can conduct of the water molecules (oxygen atom), while the
electricity. negative ions are attracted to the positive ends of
water molecules (hydrogen atom). The attraction
Ions are in fixed positions.
Ions are free to move. between the water molecules and the ions of the
They cannot move freely.
compounds are strong enough to overcome the
electrostatic attraction forces between the ions.
2δ – Na +
O
H H
δ+ δ+
Cl –
Solid state Aqueous or molten state
Figure 5.33 Electrical conductivity of ionic compounds Melting Point and Boiling Point
3. However, covalent compounds consist of neutral
molecules. They do not contain ions. Hence, 1. Ionic compounds consist of positive ions and
covalent compounds do not conduct electricity negative ions which are held together by strong
in all states. electrostatic attraction forces.
5.6.2 5.7.1 131