Page 154 - Basic Principles of Textile Coloration
P. 154
WATER SOFTENING 143
water has been demineralised. It may, however, still contain organic material and
dissolved carbon dioxide from the reaction of carbonate and bicarbonate with the
acid from the resin. A thorough aeration eliminates the carbon dioxide.
Demineralisation is important for water fed to very high pressure boilers.
The use of ion exchange resins for water treatment is relatively simple. The
resin is packed into a column containing water and treatment simply involves
flowing water up or down the column. The capacity of the resin and the ionic
content of the water determine when regeneration will be required. One problem
with beds of ion exchangers is the retention in the column of suspended matter
and living organisms in the water. Countercurrent rinsing and occasional
treatment with a bactericide minimise these problems. For removal of both cations
and anions (demineralisation), two columns in series are used, the first for strong
acid exchange and the second for strong base exchange. It is even possible to mix
anion and cation exchangers in the same bed. If the different types of particles
have different densities, they can be separated by sedimentation in a counterflow
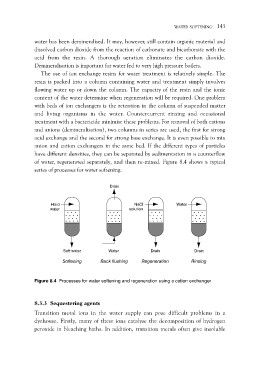
of water, regenerated separately, and then re-mixed. Figure 8.4 shows a typical
series of processes for water softening.
Drain
Hard .............. NaCl Water
water .............. solution .............. ..............
Soft water Water Drain Drain
Softening Back flushing Regeneration Rinsing
Figure 8.4 Processes for water softening and regeneration using a cation exchanger
8.3.3 Sequestering agents
Transition metal ions in the water supply can pose difficult problems in a
dyehouse. Firstly, many of these ions catalyse the decomposition of hydrogen
peroxide in bleaching baths. In addition, transition metals often give insoluble