Page 152 - Basic Principles of Textile Coloration
P. 152
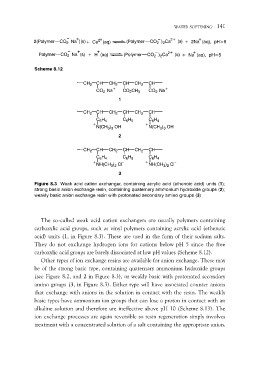
2(Polymer CO2 Na )(s)+ Ca2 (aq) WATER SOFTENING 141
Polymer CO2 Na (s) + H (aq)
(Polymer CO2 )2Ca2 (s) + 2Na (aq), pH>5
Scheme 8.12 (Polymer CO2 )2Ca2 (s) + Na (aq), pH<5
CH2 CH CH2 CH CH2 CH
CO2–Na+ CO2CH3 CO2–Na+
1
CH2 CH CH2 CH CH2 CH
C6H4 C6H5 C6H4
N(CH3)3 OH–
N(CH3)3 OH–
2
CH2 CH CH2 CH CH2 CH
C6H4 C6H5 C6H4
NH(CH3)2 Cl–
NH(CH3)2 Cl–
3
Figure 8.3 Weak acid cation exchanger, containing acrylic acid (ethenoic acid) units (1);
strong basic anion exchange resin, containing quaternary ammonium hydroxide groups (2);
weakly basic anion exchange resin with protonated secondary amino groups (3)
The so-called weak acid cation exchangers are usually polymers containing
carboxylic acid groups, such as vinyl polymers containing acrylic acid (ethenoic
acid) units (1, in Figure 8.3). These are used in the form of their sodium salts.
They do not exchange hydrogen ions for cations below pH 5 since the free
carboxylic acid groups are barely dissociated at low pH values (Scheme 8.12).
Other types of ion exchange resins are available for anion exchange. These may
be of the strong basic type, containing quaternary ammonium hydroxide groups
(see Figure 8.2, and 2 in Figure 8.3), or weakly basic with protonated secondary
amino groups (3, in Figure 8.3). Either type will have associated counter anions
that exchange with anions in the solution in contact with the resin. The weakly
basic types have ammonium ion groups that can lose a proton in contact with an
alkaline solution and therefore are ineffective above pH 10 (Scheme 8.13). The
ion exchange processes are again reversible so resin regeneration simply involves
treatment with a concentrated solution of a salt containing the appropriate anion.