Page 151 - Basic Principles of Textile Coloration
P. 151
140 WATER TREATMENT
The chemical structures of aluminosilicates are based on the structure of silica.
This consists of a three dimensional network of SiO4 units, in which the oxygen
atoms have a tetrahedral arrangement around the central silicon atom. These
tetrahedra may have common corners or faces. In an aluminosilicate, a number of
aluminium atoms replace silicon atoms in the silica structure. The aluminium
atoms are bonded to four tetrahedral oxygen atoms but because their atomic
number is one less than silicon, each aluminium atom introduced has a negative
charge, balanced by incorporation of a cation such as Na+ or K+. It is these
cations that are available for exchange.
The newer synthetic polymer ion exchangers are much more versatile than the
zeolites and are widely used for water softening and demineralisation. They are
often called ion exchange resins. Many are based on polystyrene that has been
partly crosslinked by incorporation of a small amount of divinylbenzene (2–10%).
Suspension polymerisation of the styrene and divinylbenzene produces the
crosslinked polymer in the form of small beads. These have the appearance of a
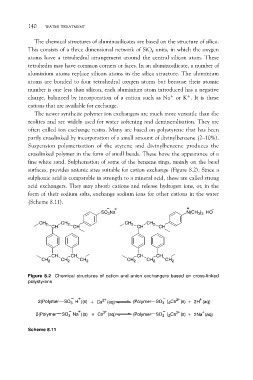
fine white sand. Sulphonation of some of the benzene rings, mainly on the bead
surfaces, provides anionic sites suitable for cation exchange (Figure 8.2). Since a
sulphonic acid is comparable in strength to a mineral acid, these are called strong
acid exchangers. They may absorb cations and release hydrogen ions, or, in the
form of their sodium salts, exchange sodium ions for other cations in the water
(Scheme 8.11).
SO3Na N(CH3)3 HO
CH2 CH CH2 CH CH2 CH CH2 CH
CH CH CH CH
CH2 CH2 CH2 CH2 CH2 CH2
Figure 8.2 Chemical structures of cation and anion exchangers based on cross-linked
polystyrene
2(Polymer SO3 H )(s) + Ca2 (aq) (Polymer SO3 )2Ca2 (s) + 2H (aq)
2(Polymer SO3 Na )(s) + Ca2 (aq) (Polymer SO3 )2Ca2 (s) + 2Na (aq)
Scheme 8.11