Page 170 - Basic Principles of Textile Coloration
P. 170
SURFACE ACTIVITY OF DETERGENTS 159
Micelle
Molecules at
the surface
Isolated molecule
Key
CH3(CH2)16—CO2–
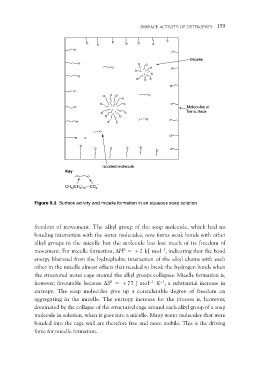
Figure 9.3 Surface activity and micelle formation in an aqueous soap solution
freedom of movement. The alkyl group of the soap molecule, which had no
bonding interaction with the water molecules, now forms weak bonds with other
alkyl groups in the micelle but the molecule has lost much of its freedom of
movement. For micelle formation, DH0 = +2 kJ mol–1, indicating that the bond
energy liberated from the hydrophobic interaction of the alkyl chains with each
other in the micelle almost offsets that needed to break the hydrogen bonds when
the structured water cage around the alkyl groups collapses. Micelle formation is,
however, favourable because DS0 = +77 J mol–1 K–1, a substantial increase in
entropy. The soap molecules give up a considerable degree of freedom on
aggregating in the micelle. The entropy increase for the process is, however,
dominated by the collapse of the structured cage around each alkyl group of a soap
molecule in solution, when it goes into a micelle. Many water molecules that were
bonded into the cage wall are therefore free and more mobile. This is the driving
force for micelle formation.