Page 171 - Basic Principles of Textile Coloration
P. 171
Property160 AUXILIARY CHEMICALS FOR WET PROCESSING AND DYEING
The formation of micelles is an important facet of surface activity. It occurs quite
suddenly once the phase boundaries are saturated with a monolayer of surfactant
molecules and the total concentration in solution increases above a critical value
called the critical micelle concentration or CMC. The CMC is a characteristic
property of a given surfactant. Its value is often lower than 1.0 g l–1. The formation
of micelles depends on:
(1) the preference of the surfactant alkyl groups to interact with each other, in
their own hydrophobic environment, rather than remain exposed to the
water;
(2) the freeing of water molecules from the hydrogen-bonded cage around the
hydrophobic groups when the surfactant molecules aggregate together.
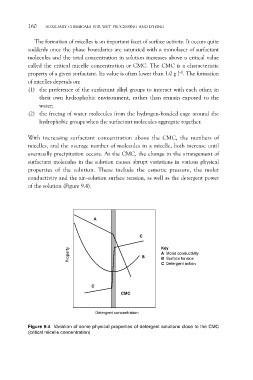
With increasing surfactant concentration above the CMC, the numbers of
micelles, and the average number of molecules in a micelle, both increase until
eventually precipitation occurs. At the CMC, the change in the arrangement of
surfactant molecules in the solution causes abrupt variations in various physical
properties of the solution. These include the osmotic pressure, the molar
conductivity and the air–solution surface tension, as well as the detergent power
of the solution (Figure 9.4).
A
C
Key
A Molar conductivity
B B Surface tension
C Detergent action
C
CMC
Detergent concentration
Figure 9.4 Variation of some physical properties of detergent solutions close to the CMC
(critical micelle concentration)