Page 199 - Basic Principles of Textile Coloration
P. 199
Exhaustion/%188 AN INTRODUCTION TO DYES AND DYEING
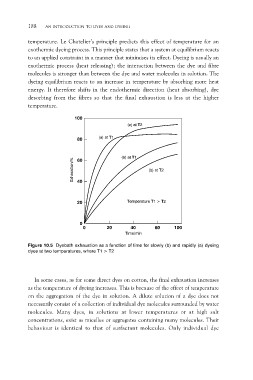
temperature. Le Chatelier’s principle predicts this effect of temperature for an
exothermic dyeing process. This principle states that a system at equilibrium reacts
to an applied constraint in a manner that minimises its effect. Dyeing is usually an
exothermic process (heat releasing); the interaction between the dye and fibre
molecules is stronger than between the dye and water molecules in solution. The
dyeing equilibrium reacts to an increase in temperature by absorbing more heat
energy. It therefore shifts in the endothermic direction (heat absorbing), dye
desorbing from the fibres so that the final exhaustion is less at the higher
temperature.
100
(a) at T2
80 (a) at T1
(b) at T1
60
(b) at T2
40
20 Temperature T1 > T2
0
0 20 40 60 100
Time/min
Figure 10.5 Dyebath exhaustion as a function of time for slowly (b) and rapidly (a) dyeing
dyes at two temperatures, where T1 > T2
In some cases, as for some direct dyes on cotton, the final exhaustion increases
as the temperature of dyeing increases. This is because of the effect of temperature
on the aggregation of the dye in solution. A dilute solution of a dye does not
necessarily consist of a collection of individual dye molecules surrounded by water
molecules. Many dyes, in solutions at lower temperatures or at high salt
concentrations, exist as micelles or aggregates containing many molecules. Their
behaviour is identical to that of surfactant molecules. Only individual dye