Page 48 - Basic Principles of Textile Coloration
P. 48
POLYMER STRUCTURE 37
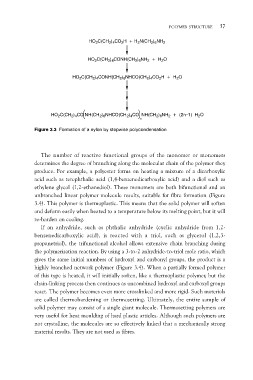
HO2C(CH2)4CO2H + H2N(CH2)6NH2
HO2C(CH2)4CONH(CH2)6NH2 + H2O
HO2C(CH2)4CONH(CH2)6NHCO(CH2)4CO2H + H2O
HO2C(CH2)4CO NH(CH2)6NHCO(CH2)4CO NH(CH2)6NH2 + (2n–1) H2O
n
Figure 3.3 Formation of a nylon by stepwise polycondensation
The number of reactive functional groups of the monomer or monomers
determines the degree of branching along the molecular chain of the polymer they
produce. For example, a polyester forms on heating a mixture of a dicarboxylic
acid such as terephthalic acid (1,4-benzenedicarboxylic acid) and a diol such as
ethylene glycol (1,2-ethanediol). These monomers are both bifunctional and an
unbranched linear polymer molecule results, suitable for fibre formation (Figure
3.4). This polymer is thermoplastic. This means that the solid polymer will soften
and deform easily when heated to a temperature below its melting point, but it will
re-harden on cooling.
If an anhydride, such as phthalic anhydride (cyclic anhydride from 1,2-
benzenedicarboxylic acid), is reacted with a triol, such as glycerol (1,2,3-
propanetriol), the trifunctional alcohol allows extensive chain branching during
the polymerisation reaction. By using a 3-to-2 anhydride-to-triol mole ratio, which
gives the same initial numbers of hydroxyl and carbonyl groups, the product is a
highly branched network polymer (Figure 3.4). When a partially formed polymer
of this type is heated, it will initially soften, like a thermoplastic polymer, but the
chain-linking process then continues as uncombined hydroxyl and carboxyl groups
react. The polymer becomes even more crosslinked and more rigid. Such materials
are called thermohardening or thermosetting. Ultimately, the entire sample of
solid polymer may consist of a single giant molecule. Thermosetting polymers are
very useful for heat moulding of hard plastic articles. Although such polymers are
not crystalline, the molecules are so effectively linked that a mechanically strong
material results. They are not used as fibres.