Page 55 - Basic Principles of Textile Coloration
P. 55
44 FIBROUS POLYMERS
dipole–dipole attraction between the cyano groups in polyacrylonitrile. In some
cases, molecules with permanent dipoles can exert attractive forces on
neighbouring non-polar molecules by polarising their electrons. The
electropositive end of a dipole attracts and polarises the electrons in a bond
between two atoms in a neighbouring molecule while the electronegative end of
the dipole becomes attracted to the neighbouring atom’s more exposed nucleus.
Dipole–dipole and dipole-induced dipole bonds are weak and only significant
when molecules are in close contact. They are therefore continually being broken
and reformed as molecules collide.
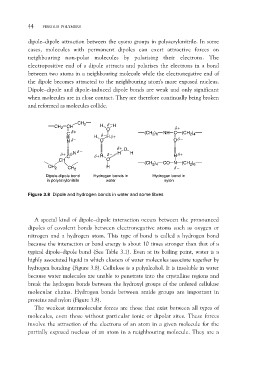
CH2 CH CH2 H δ _H δ+
C δ+ O (CH2)6 NH C (CH2)4
Nδ_
H δ _Hδ+ Oδ _
O
δ+C Nδ _ δ+H δ _ δ+ O Hδ+
CH O HH CO Nδ _ (CH2)6
H
CH2 CH2 (CH2)4
Dipole-dipole bond Hydrogen bonds in Hydrogen bond in
in polyacrylonitrile water nylon
Figure 3.8 Dipole and hydrogen bonds in water and some fibres
A special kind of dipole–dipole interaction occurs between the pronounced
dipoles of covalent bonds between electronegative atoms such as oxygen or
nitrogen and a hydrogen atom. This type of bond is called a hydrogen bond
because the interaction or bond energy is about 10 times stronger than that of a
typical dipole–dipole bond (See Table 3.1). Even at its boiling point, water is a
highly associated liquid in which clusters of water molecules associate together by
hydrogen bonding (Figure 3.8). Cellulose is a polyalcohol. It is insoluble in water
because water molecules are unable to penetrate into the crystalline regions and
break the hydrogen bonds between the hydroxyl groups of the ordered cellulose
molecular chains. Hydrogen bonds between amide groups are important in
proteins and nylon (Figure 3.8).
The weakest intermolecular forces are those that exist between all types of
molecules, even those without particular ionic or dipolar sites. These forces
involve the attraction of the electrons of an atom in a given molecule for the
partially exposed nucleus of an atom in a neighbouring molecule. They are a