Page 54 - Basic Principles of Textile Coloration
P. 54
INTERMOLECULAR FORCES 43
forces are responsible for many phenomena in textile chemistry, including the
coherence of the crystalline regions in fibres, and even the dyeing process itself.
Ionic bonds are the simplest to understand since they involve the attraction of
oppositely charged ions that have atoms with more or less than the required
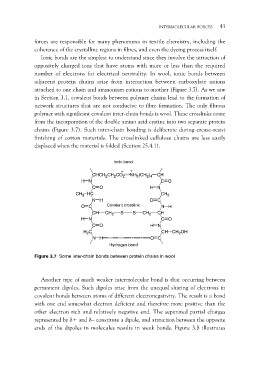
number of electrons for electrical neutrality. In wool, ionic bonds between
adjacent protein chains arise from interaction between carboxylate anions
attached to one chain and ammonium cations to another (Figure 3.7). As we saw
in Section 3.1, covalent bonds between polymer chains lead to the formation of
network structures that are not conducive to fibre formation. The only fibrous
polymer with significant covalent inter-chain bonds is wool. These crosslinks come
from the incorporation of the double amino acid cystine into two separate protein
chains (Figure 3.7). Such inter-chain bonding is deliberate during crease-resist
finishing of cotton materials. The crosslinked cellulose chains are less easily
displaced when the material is folded (Section 25.4.1).
Ionic bond
CHCH2CH2CO2 NH3(CH2)4 CH
HN CO
CO HN
CH3 HC CH2
OC
NH
NH
OC Covalent crosslink
CH CH2 S S CH2 CH
HN CO
CO HN
H2C CH CH2OH
NH OC
Hydrogen bond
Figure 3.7 Some inter-chain bonds between protein chains in wool
Another type of much weaker intermolecular bond is that occurring between
permanent dipoles. Such dipoles arise from the unequal sharing of electrons in
covalent bonds between atoms of different electronegativity. The result is a bond
with one end somewhat electron deficient and therefore more positive than the
other electron rich and relatively negative end. The separated partial charges
represented by d+ and d– constitute a dipole, and attraction between the opposite
ends of the dipoles in molecules results in weak bonds. Figure 3.8 illustrates