Page 615 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 615
CHAPTER 50: Novel Modes of Mechanical Ventilation 435
risk for VILI increases as end inspiratory transpulmonary pressures . .
exceed 30 to 35 cm H O, as tidal volumes exceed 8 to 10 mL/kg (ideal V V
2
body weight), and as regions of repetitive alveolar opening-closing
develop. 2-12 Other ventilatory pattern factors may also be involved in
the development of VILI. These include frequency of stretch and the Flow
13
acceleration/velocity of stretch. 14
Importantly, VILI is associated with cytokine release and bacterial V V
4-6
translocation. These are often implicated as important contributors T T
15
to the systemic inflammatory response with multiorgan dysfunction
that results in VILI associated mortality. The incidence of VILI has Volume
been reported to be as high as 24% of patients who are receiving
mechanical ventilation for reasons other than ALI/ARDS although
estimates widely vary. 5,7,16 P AW P AW
Another conceptual source of injury during mechanical ventilatory
support is oxygen toxicity. Oxygen concentrations approaching 100%
are known to cause oxidant injuries in airways and lung parenchyma. Pressure
17
A “safe” oxygen concentration or duration of exposure is not clear in 2 4 6 2 4 6
sick humans, however, since most of the data supporting the concept
of oxygen toxicity comes from animals. Most consensus groups have
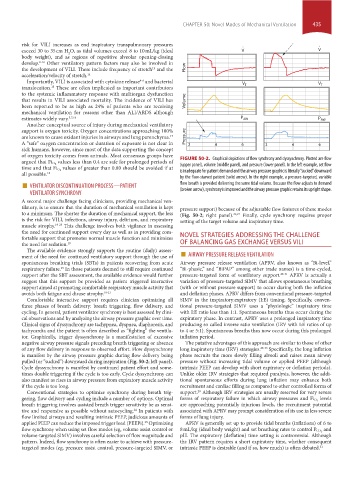
values less than 0.4 are safe for prolonged periods of FIGURE 50-2. Graphical depictions of flow synchrony and dyssynchrony. Plotted are flow
argued that Fi O 2 (upper panel), volume (middle panel), and pressure (lower panel). In the left example, set flow
values of greater than 0.80 should be avoided if at
time and that Fi O 2 is inadequate for patient demand and the airway pressure graphic is literally “sucked” downward
all possible. 18 by the flow-starved patient (solid arrow). In the right example, a pressure-targeted, variable
■ VENTILATOR DISCONTINUATION PROCESS—PATIENT flow breath is provided delivering the same tidal volume. Because the flow adjusts to demand
VENTILATOR SYNCHRONY (broken arrow), synchrony is improved and the airway pressure graphic retains its upright shape.
A second major challenge facing clinicians, providing mechanical ven-
tilatory, is to ensure that the duration of mechanical ventilation is kept pressure support) because of the adjustable flow features of these modes
to a minimum. The shorter the duration of mechanical support, the less (Fig. 50-2; right panel). 26,27 Finally, cycle synchrony requires proper
is the risk for VILI, infections, airway injury, delirium, and respiratory setting of the target volume and inspiratory time.
muscle atrophy. 19-23 This challenge involves both vigilance in assessing
the need for continued support every day as well as in providing com-
fortable support that promotes normal muscle function and minimizes NOVEL STRATEGIES ADDRESSING THE CHALLENGE
the need for sedation. 19 OF BALANCING GAS EXCHANGE VERSUS VILI
ment of the need for continued ventilatory support through the use of ■ AIRWAY PRESSURE RELEASE VENTILATION
The available evidence strongly supports the routine (daily) assess-
spontaneous breathing trials (SBTs) in patients recovering from acute Airway pressure release ventilation (APRV, also known as “Bi-level,”
respiratory failure. In those patients deemed to still require continued “Bi-phasic,” and “BiPAP” among other trade names) is a time-cycled,
19
support after the SBT assessment, the available evidence would further pressure-targeted form of ventilatory support. 28-31 APRV is actually a
suggest that this support be provided as patient triggered interactive variation of pressure-targeted SIMV that allows spontaneous breathing
support aimed at promoting comfortable respiratory muscle activity that (with or without pressure support) to occur during both the inflation
avoids both fatigue and disuse atrophy. 19-23 and deflation phases. APRV differs from conventional pressure-targeted
Comfortable interactive support requires clinician optimizing all SIMV in the inspiratory:expiratory (I:E) timing. Specifically, conven-
three phases of breath delivery: breath triggering, flow delivery, and tional pressure-targeted SIMV uses a “physiologic” inspiratory time
cycling. In general, patient ventilator synchrony is best assessed by clini- with I:E ratio less than 1:1. Spontaneous breaths thus occur during the
cal observations and by analyzing the airway pressure graphic over time. expiratory phase. In contrast, APRV uses a prolonged inspiratory time
Clinical signs of dyssynchrony are tachypnea, dyspnea, diaphoresis, and producing so called inverse ratio ventilation (IRV with I:E ratios of up
tachycardia and the patient is often described as “fighting” the ventila- to 4 or 5:1). Spontaneous breaths thus now occur during this prolonged
tor. Graphically, trigger dyssynchrony is a manifestation of excessive inflation period.
negative airway pressure signals preceding breath triggering or absence The putative advantages of this approach are similar to those of other
of any flow delivery in response to observed effort. Flow dyssynchrony long inspiratory time (IRV) strategies. 28-33 Specifically, the long inflation
is manifest by the airway pressure graphic during flow delivery being phase recruits the more slowly filling alveoli and raises mean airway
pulled (or “sucked”) downward during inspiration (Fig. 50-2; left panel). pressure without increasing tidal volume or applied PEEP (although
Cycle dyssynchrony is manifest by continued patient effort and some- intrinsic PEEP can develop with short expiratory or deflation periods).
times double triggering if the cycle is too early. Cycle dyssynchrony can Unlike older IRV strategies that required paralysis, however, the addi-
also manifest as rises in airway pressure from expiratory muscle activity tional spontaneous efforts during lung inflation may enhance both
if the cycle is too long. recruitment and cardiac filling as compared to other controlled forms of
Conventional strategies to optimize synchrony during breath trig- support. Although IRV strategies are usually reserved for very severe
28
gering, flow delivery and cycling include a number of options. Optimal forms of respiratory failure in which airway pressures and Fi O 2 levels
breath triggering involves assisted breath trigger sensitivity be as sensi- are approaching potentially injurious levels, the recruitment potential
tive and responsive as possible without autocycling. In patients with associated with APRV may prompt consideration of its use in less severe
24
flow limited airways and resulting intrinsic PEEP, judicious amounts of forms of lung injury.
applied PEEP can reduce the imposed trigger load (PEEPi). Optimizing APRV is generally set up to provide tidal breaths (inflations) of 6 to
25
flow synchrony when using set flow modes (eg, volume assist control or 8 mL/kg (ideal body weight) and set breathing rates to control P CO 2 and
volume-targeted SIMV) involves careful selection of flow magnitude and pH. The expiratory (deflation) time setting is controversial. Although
pattern. Indeed, flow synchrony is often easier to achieve with pressure- the IRV pattern requires a short expiratory time, whether consequent
targeted modes (eg, pressure assist control, pressure-targeted SIMV, or intrinsic PEEP is desirable (and if so, how much) is often debated. 31
section04.indd 435 1/23/2015 2:19:23 PM