Page 1190 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1190
1038 Part VII Hematologic Malignancies
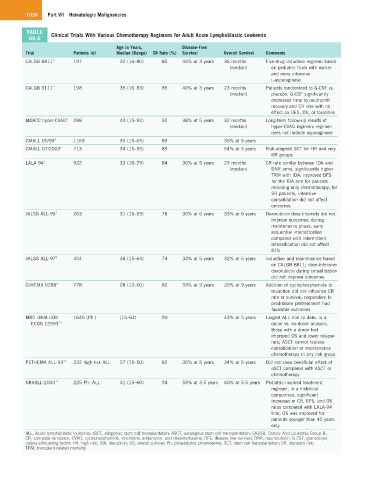
TABLE Clinical Trials With Various Chemotherapy Regimens for Adult Acute Lymphoblastic Leukemia
66.6
Age in Years, Disease-Free
Trial Patients (n) Median (Range) CR Rate (%) Survival Overall Survival Comments
CALGB 8811 1 197 32 (16–80) 85 46% at 3 years 36 months Five-drug induction regimen based
(median) on pediatric trials with earlier
and more intensive
L-asparaginase
CALGB 9111 2 198 35 (16–83) 85 40% at 3 years 23 months Patients randomized to G-CSF vs.
(median) placebo; G-CSF significantly
decreased time to neutrophil
recovery and CR rate with no
effect on DFS, OS, or toxicities
MDACC hyper-CVAD 3 288 40 (15–92) 92 38% at 5 years 32 months Long-term follow-up results of
(median) hyper-CVAD regimen; regimen
does not include asparaginase
GMALL 05/93 4 1163 35 (15–65) 83 35% at 5 years
GMALL 07/2003 5 713 34 (15–55) 89 54% at 5 years Risk-adapted SCT for HR and very
HR groups
LALA 94 6 922 33 (18–79) 84 30% at 5 years 23 months CR rate similar between IDA and
(median) DNR arms; significantly higher
TRM with IDA; improved DFS
for the IDA arm for patients
receiving only chemotherapy; for
SR patients, intensive
consolidation did not affect
outcomes
JALSG ALL-93 7 263 31 (15–59) 78 30% at 6 years 33% at 6 years Doxorubicin dose intensity did not
improve outcomes; during
maintenance phase, early
sequential intensification
compared with intermittent
intensification did not affect
DFS
JALSG ALL-97 8 404 38 (15–64) 74 33% at 5 years 32% at 5 years Induction and maintenance based
on CALGB 8811; dose-intensive
doxorubicin during consolidation
did not improve outcomes
GIMEMA 0288 9 778 28 (12–60) 82 33% at 9 years 29% at 9 years Addition of cyclophosphamide to
induction did not influence CR
rate or survival; responders to
prednisone pretreatment had
favorable outcomes
MRC UKALLXII/ 1646 (Ph ) – (15–64) 90 43% at 5 years Largest ALL trial to date; in a
ECOG E2993 10 donor vs. no-donor analysis,
those with a donor had
improved OS and lower relapse
rate; ASCT cannot replace
consolidation or maintenance
chemotherapy in any risk group
PETHEMA ALL-93 11 222 high-risk ALL 27 (15–50) 82 35% at 5 years 34% at 5 years Did not show beneficial effect of
aSCT compared with ASCT or
chemotherapy
GRAALL-2003 12 225 Ph- ALL 31 (15–60) 94 59% at 3.5 years 60% at 3.5 years Pediatric-inspired treatment
regimen; in a historical
comparison, significant
increases in CR, EFS, and OS
rates compared with LALA-94
trial; OS was improved for
patients younger than 45 years
only
ALL, Acute lymphoblastic leukemia; aSCT, allogeneic stem cell transplantation; ASCT, autologous stem cell transplantation; CALGB, Cancer And Leukemia Group B;
CR, complete remission; CVAD, cyclophosphamide, vincristine, adriamycin, and dexamethasone; DFS, disease-free survival; DNR, daunorubicin; G-CSF, granulocyte
colony-stimulating factor; HR, high risk; IDA, idarubicin; OS, overall survival; Ph, philadelphia chromosome; SCT, stem cell transplantation; SR, standard risk;
TRM, transplant-related mortality.