Page 1291 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1291
Chapter 70 Primary Myelofibrosis 1137
C
A B D E
F G H I
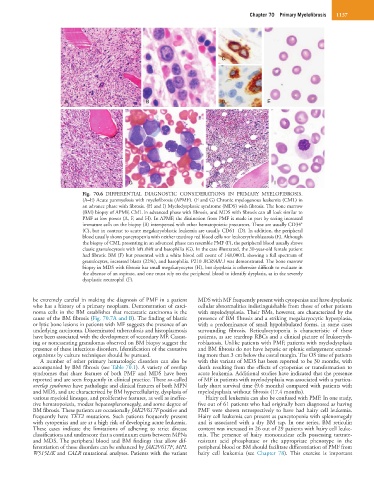
Fig. 70.6 DIFFERENTIAL DIAGNOSTIC CONSIDERATIONS IN PRIMARY MYELOFIBROSIS.
(A–E) Acute panmyelosis with myelofibrosis (APMF). (F and G) Chronic myelogenous leukemia (CML) in
an advance phase with fibrosis. (H and I) Myelodysplastic syndrome (MDS) with fibrosis. The bone marrow
(BM) biopsy of APMF, CML in advanced phase with fibrosis, and MDS with fibrosis can all look similar to
PMF at low power (A, F, and H). In APMF, the distinction from PMF is made in part by seeing increased
+
immature cells on the biopsy (B) interspersed with other hematopoietic precursors. These are usually CD34
–
(C), but in contrast to acute megakaryoblastic leukemia are usually CD61 (D). In addition, the peripheral
blood usually shows pancytopenia with neither teardrop red blood cells nor leukoerythroblastosis (E). Although
the biopsy of CML presenting in an advanced phase can resemble PMF (F), the peripheral blood usually shows
classic granulocytosis with left shift and basophilia (G). In the case illustrated, the 30-year-old female patient
had fibrotic BM (F) but presented with a white blood cell count of 148,000/L showing a full spectrum of
granulocytes, increased blasts (22%), and basophilia. P210 BCR/ABL1 was demonstrated. The bone marrow
biopsy in MDS with fibrosis has small megakaryocytes (H), but dysplasia is otherwise difficult to evaluate in
the absence of an aspirate, and one must rely on the peripheral blood to identify dysplasia, as in the severely
dysplastic neutrophil (F).
be extremely careful in making the diagnosis of PMF in a patient MDS with MF frequently present with cytopenias and have dysplastic
who has a history of a primary neoplasm. Demonstration of carci- cellular abnormalities indistinguishable from those of other patients
noma cells in the BM establishes that metastatic carcinoma is the with myelodysplasia. Their BMs, however, are characterized by the
cause of the BM fibrosis (Fig. 70.7A and B). The finding of blastic presence of BM fibrosis and a striking megakaryocytic hyperplasia,
or lytic bone lesions in patients with MF suggests the presence of an with a predominance of small hypolobulated forms, in some cases
underlying carcinoma. Disseminated tuberculosis and histoplasmosis surrounding fibrosis. Reticulocytopenia is characteristic of these
have been associated with the development of secondary MF. Caseat- patients, as are teardrop RBCs and a clinical picture of leukoeryth-
ing or noncaseating granulomas observed on BM biopsy suggest the roblastosis. Unlike patients with PMF, patients with myelodysplasia
presence of these infectious disorders. Identification of the causative and BM fibrosis do not have hepatic or splenic enlargement extend-
organisms by culture techniques should be pursued. ing more than 3 cm below the costal margin. The OS time of patients
A number of other primary hematologic disorders can also be with this variant of MDS has been reported to be 30 months, with
accompanied by BM fibrosis (see Table 70.1). A variety of overlap death resulting from the effects of cytopenias or transformation to
syndromes that share features of both PMF and MDS have been acute leukemia. Additional studies have indicated that the presence
reported and are seen frequently in clinical practice. These so-called of MF in patients with myelodysplasia was associated with a particu-
overlap syndromes have pathologic and clinical features of both MPN larly short survival time (9.6 months) compared with patients with
and MDS, and are characterized by BM hypercellularity, dysplasia of myelodysplasia without fibrosis (17.4 months).
various myeloid lineages, and proliferative features, as well as ineffec- Hairy cell leukemia can also be confused with PMF. In one study,
tive hematopoiesis, modest hepatosplenomegaly, and some degree of five out of 61 patients who had originally been diagnosed as having
BM fibrosis. These patients are occasionally JAK2V617F positive and PMF were shown retrospectively to have had hairy cell leukemia.
frequently have TET2 mutations. Such patients frequently present Hairy cell leukemia can present as pancytopenia with splenomegaly
with cytopenias and are at a high risk of developing acute leukemia. and is associated with a dry BM tap. In one series, BM reticulin
These cases indicate the limitations of adhering to strict disease content was increased in 26 out of 29 patients with hairy cell leuke-
classifications and underscore that a continuum exists between MPNs mia. The presence of hairy mononuclear cells possessing tartrate-
and MDS. The peripheral blood and BM findings that allow dif- resistant acid phosphatase or the appropriate phenotype in the
ferentiation of these disorders can be enhanced by JAK2V617F, MPL peripheral blood or BM should facilitate differentiation of PMF from
W515L/K and CALR mutational analyses. Patients with the variant hairy cell leukemia (see Chapter 78). This exercise is important