Page 1485 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1485
1320 Part VII Hematologic Malignancies
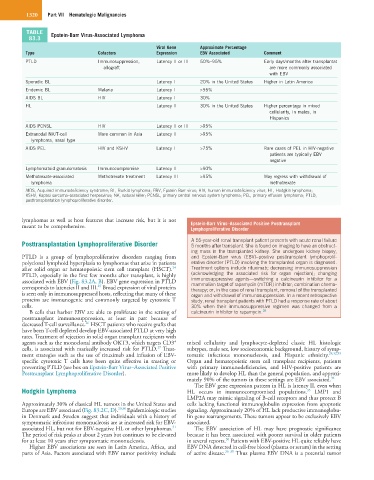
TABLE Epstein-Barr Virus-Associated Lymphoma
83.3
Viral Gene Approximate Percentage
Type Cofactors Expression EBV Associated Comment
PTLD Immunosuppression, Latency II or III 50%–95% Early days/months after transplantat
allograft are more commonly associated
with EBV
Sporadic BL Latency I 20% in the United States Higher in Latin America
Endemic BL Malaria Latency I >95%
AIDS BL HIV Latency I 30%
HL Latency II 30% in the United States Higher percentage in mixed
cellularity, in males, in
Hispanics
AIDS PCNSL HIV Latency II or III >95%
Extranodal NK/T-cell More common in Asia Latency II >95%
lymphoma, nasal type
AIDS PEL HIV and KSHV Latency I >75% Rare cases of PEL in HIV-negative
patients are typically EBV
negative
Lymphomatoid granulomatosis Immunocompromise Latency II >90%
Methotrexate-associated Methotrexate treatment Latency III >95% May regress with withdrawal of
lymphoma methotrexate
AIDS, Acquired immunodeficiency syndrome; BL, Burkitt lymphoma; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; HL, Hodgkin lymphoma;
KSHV, Kaposi sarcoma–associated herpesvirus; NK, natural killer; PCNSL, primary central nervous system lymphoma; PEL, primary effusion lymphoma; PTLD,
posttransplantation lymphoproliferative disorder.
lymphomas as well as host features that increase risk, but it is not
meant to be comprehensive. Epstein-Barr Virus–Associated Positive Posttransplant
Lymphoproliferative Disorder
Posttransplantation Lymphoproliferative Disorder A 55-year-old renal transplant patient presents with acute renal failure
5 months after transplant. She is found on imaging to have an obstruct-
ing mass in the transplanted kidney. She undergoes kidney biopsy,
PTLD is a group of lymphoproliferative disorders ranging from and Epstein-Barr virus (EBV)–positive posttransplant lymphoprolif-
polyclonal lymphoid hyperplasia to lymphomas that arise in patients erative disorder (PTLD) involving the transplanted organ is diagnosed.
24
after solid organ or hematopoietic stem cell transplant (HSCT). Treatment options include rituximab; decreasing immunosuppression
PTLD, especially in the first few months after transplant, is highly (acknowledging the associated risk for organ rejection); changing
associated with EBV (Fig. 83.2A, B). EBV gene expression in PTLD immunosuppressive agents—switching a calcineurin inhibitor for a
mammalian target of rapamycin (mTOR) inhibitor; combination chemo-
25
corresponds to latencies II and III. Broad expression of viral proteins therapy; or, in the case of renal transplant, removal of the transplanted
is seen only in immunosuppressed hosts, reflecting that many of these organ and withdrawal of immunosuppression. In a recent retrospective
proteins are immunogenic and commonly targeted by cytotoxic T study, renal transplant patients with PTLD had a response rate of about
cells. 30% when their immunosuppressive regimen was changed from a
B cells that harbor EBV are able to proliferate in the setting of calcineurin inhibitor to rapamycin. 28
posttransplant immunosuppression, at least in part because of
26
decreased T-cell surveillance. HSCT patients who receive grafts that
have been T-cell depleted develop EBV-associated PTLD at very high
rates. Treatment of rejection in solid organ transplant recipients with
+
agents such as the monoclonal antibody OKT3, which targets CD3 mixed cellularity and lymphocyte-depleted classic HL histologic
27
cells, is associated with markedly increased risk for PTLD. Treat- subtypes, male sex, low socioeconomic background, history of symp-
ment strategies such as the use of rituximab and infusion of EBV- tomatic infectious mononucleosis, and Hispanic ethnicity. 29,32,33
specific cytotoxic T cells have been quite effective in treating or Organ and hematopoietic stem cell transplant recipients, patients
preventing PTLD (see box on Epstein-Barr Virus–Associated Positive with primary immunodeficiencies, and HIV-positive patients are
Posttransplant Lymphoproliferative Disorder). more likely to develop HL than the general population, and approxi-
mately 90% of the tumors in these settings are EBV associated. 34
The EBV gene expression pattern in HL is latency II, even when
Hodgkin Lymphoma HL occurs in immunocompromised populations. LMP1 and
35
LMP2A may mimic signaling of B-cell receptors and thus protect B
Approximately 30% of classical HL tumors in the United States and cells lacking functional immunoglobulin expression from apoptotic
Europe are EBV associated (Fig. 83.2C, D). 29,30 Epidemiologic studies signaling. Approximately 20% of HL lack productive immunoglobu-
in Denmark and Sweden suggest that individuals with a history of lin gene rearrangements. These tumors appear to be exclusively EBV
symptomatic infectious mononucleosis are at increased risk for EBV- associated.
31
associated HL, but not for EBV-negative HL or other lymphomas. The EBV association of HL may have prognostic significance
The period of risk peaks at about 2 years but continues to be elevated because it has been associated with poorer survival in older patients
30
for at least 10 years after symptomatic mononucleosis. in several reports. Patients with EBV-positive HL quite reliably have
Higher EBV associations are seen in Latin America, Africa, and EBV DNA detected in cell-free blood (plasma or serum) in the setting
parts of Asia. Factors associated with EBV tumor positivity include of active disease. 36–38 Thus plasma EBV DNA is a potential tumor