Page 1487 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1487
1322 Part VII Hematologic Malignancies
A B C
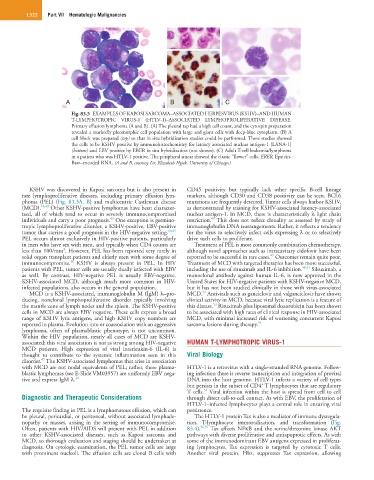
Fig. 83.3 EXAMPLES OF KAPOSI SARCOMA–ASSOCIATED HERPESVIRUS (KSHV)–AND HUMAN
T-LYMPHOTROPIC VIRUS-1 (HTLV-1)–ASSOCIATED LYMPHOPROLIFERATIVE DISEASE.
Primary effusion lymphoma (A and B). (A) The pleural tap had a high cell count, and the cytospin preparation
revealed a markedly pleomorphic cell population with large and giant cells with deep-blue cytoplasm. (B) A
cell block was prepared (top) so that in situ hybridization studies could be performed. These studies showed
the cells to be KSHV positive by immunohistochemistry for latency associated nuclear antigen-1 (LANA-1)
(bottom) and EBV positive by EBER in situ hybridization (not shown). (C) Adult T-cell leukemia/lymphoma
in a patient who was HTLV-1 positive. The peripheral smear showed the classic “flower” cells. EBER, Epstein-
Barr–encoded RNA. (A and B, courtesy Dr. Elizabeth Hyjek, University of Chicago.)
KSHV was discovered in Kaposi sarcoma but is also present in CD45 positivity but typically lack other specific B-cell lineage
rare lymphoproliferative diseases, including primary effusion lym- markers, although CD30 and CD38 positivity can be seen. BCL6
phoma (PEL) (Fig. 83.3A, B) and multicentric Castleman disease mutations are frequently detected. Tumor cells always harbor KSHV,
(MCD). 16,42 Other KSHV-positive lymphomas have been character- as demonstrated by staining for KSHV-associated latency-associated
ized, all of which tend to occur in severely immunocompromised nuclear antigen-1. In MCD, there is characteristically λ light chain
43
48
individuals and carry a poor prognosis. One exception is germino- restriction. This does not reflect clonality as assessed by study of
tropic lymphoproliferative disorder, a KSHV-positive, EBV-positive immunoglobulin DNA rearrangements. Rather, it reflects a tendency
tumor that carries a good prognosis in the HIV-negative setting. 44,45 for the virus to selectively infect cells expressing λ or to selectively
PEL occurs almost exclusively in HIV-positive patients, particularly drive such cells to proliferate.
in men who have sex with men, and typically when CD4 counts are Treatment of PEL is most commonly combination chemotherapy,
3
less than 100/mm . However, PEL has been reported very rarely in although novel approaches such as intracavitary cidofovir have been
49
solid organ transplant patients and elderly men with some degree of reported to be successful in rare cases. Outcomes remain quite poor.
46
immunocompromise. KSHV is always present in PEL. In HIV Treatment of MCD with targeted therapies has been more successful,
patients with PEL, tumor cells are usually dually infected with EBV including the use of rituximab and IL-6 inhibition. 50,51 Siltuximab, a
as well. By contrast, HIV-negative PEL is usually EBV-negative. monoclonal antibody against human IL-6, is now approved in the
KSHV-associated MCD, although much more common in HIV- United States for HIV-negative patients with KSHV-negative MCD,
infected populations, also occurs in the general population. but it has not been studied clinically in those with virus-associated
52
MCD is a KSHV-associated, immunoglobulin M (IgM) λ–pro- MCD. Antivirals such as ganciclovir and valganciclovir have shown
ducing, nonclonal lymphoproliferative disorder typically involving clinical activity in MCD, because viral lytic replication is a feature of
53
the mantle zone of lymph nodes and the spleen. The KSHV-positive this disease. Rituximab plus liposomal doxorubicin has been shown
cells in MCD are always EBV negative. These cells express a broad to be associated with high rates of clinical response in HIV-associated
range of KSHV lytic antigens, and high KSHV copy numbers are MCD, with minimal increased risk of worsening concurrent Kaposi
reported in plasma. Evolution into or coassociation with an aggressive sarcoma lesions during therapy. 54
lymphoma, often of plasmablastic phenotype, is not uncommon.
Within the HIV population, nearly all cases of MCD are KSHV-
associated; this viral association is not as strong among HIV-negative HUMAN T-LYMPHOTROPIC VIRUS-1
MCD patients. High expression of viral interleukin-6 (IL-6) is
thought to contribute to the systemic inflammation seen in this Viral Biology
47
disorder. The KSHV-associated lymphomas that arise in association
with MCD are not nodal equivalents of PEL; rather, these plasma- HTLV-1 is a retrovirus with a single-stranded RNA genome. Follow-
blastic lymphomas (see E-Slide VM03957) are uniformly EBV nega- ing infection there is reverse transcription and integration of proviral
tive and express IgM λ. 16 DNA into the host genome. HTLV-1 infects a variety of cell types
+
but persists in the subset of CD4 T lymphocytes that are regulatory
55
T cells. Viral infection within the host is spread from cell to cell
Diagnostic and Therapeutic Considerations through direct cell-to-cell contact. As with EBV, the proliferation of
HTLV-1–infected lymphocytes plays a central role in ensuring viral
The requisite finding in PEL is a lymphomatous effusion, which can persistence.
be pleural, pericardial, or peritoneal, without associated lymphade- The HTLV-1 protein Tax is also a mediator of immune dysregula-
nopathy or masses, arising in the setting of immunocompromise. tion, T-lymphocyte immortalization, and transformation (Fig.
Often, patients with HIV/AIDS will present with PEL in addition 83.4). 56,57 Tax affects NFκB and the serine/threonine kinase AKT
to other KSHV-associated diseases, such as Kaposi sarcoma and pathways with diverse proliferative and antiapoptotic effects. As with
MCD, so thorough evaluation and staging should be undertaken at some of the immunodominant EBV antigens expressed in proliferat-
diagnosis. On cytologic examination, the PEL tumor cells are large ing lymphocytes, Tax expression is targeted by cytotoxic T cells.
with prominent nucleoli. The effusion cells are clonal B cells with Another viral protein, Hbz, suppresses Tax expression, allowing