Page 1583 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1583
1410 Part VII Hematologic Malignancies
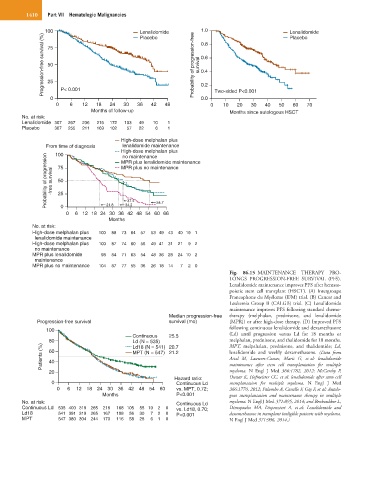
100 Lenalidomide 1.0 Lenalidomide
Progression-free survival (%) 50 Probability of progression-free survival 0.6
Placebo
Placebo
0.8
75
0.4
25
0.0
0 P< 0.001 0.2 Two-sided P<0.001
0 6 12 18 24 30 36 42 48 0 10 20 30 40 50 60 70
Months of follow-up Months since autologous HSCT
No. at risk:
Lenalidomide 307 267 236 216 172 103 49 10 1
Placebo 307 255 211 169 102 57 22 6 1
High-dose melphalan plus
From time of diagnosis lenalidomide maintenance
High-dose melphalan plus
100
Probability of progression -free survival 75 MPR plus no maintenance
no maintenance
MPR plus lenalidomide maintenance
50
25
37.4
54.7
21.8
34.2
0
0 6 12 18 24 30 36 42 48 54 60 66
Months
No. at risk:
High-dose melphalan plus 100 88 73 64 57 53 49 43 40 19 1
lenalidomide maintenance
High-dose melphalan plus 100 87 74 60 56 49 41 31 21 9 2
no maintenance
MPR plus lenalidomide 98 84 71 63 54 48 36 28 24 10 2
maintenance
MPR plus no maintenance 104 87 77 55 36 26 18 14 7 2 0
Fig. 86.15 MAINTENANCE THERAPY PRO-
LONGS PROGRESSION-FREE SURVIVAL (PFS).
Lenalidomide maintenance improves PFS after hemato-
poietic stem cell transplant (HSCT). (A) Intergroupe
Francophone du Myélome (IFM) trial. (B) Cancer and
Leukemia Group B (CALGB) trial. (C) Lenalidomide
maintenance improves PFS following standard chemo-
Median progression-free therapy (melphalan, prednisone, and lenalidomide
Progression-free survival survival (mo) [MPR]) or after high-dose therapy. (D) Improved PFS
following continuous lenalidomide and dexamethasone
100
Continuous 25.5 (Ld) until progression versus Ld for 18 months or
80 Ld (N = 535) 20.7 melphalan, prednisone, and thalidomide for 18 months.
Patients (%) 60 MPT (N = 547) 21.2 lenalidomide and weekly dexamethasone. (Data from
MPT, melpahalan, prednisone, and thalidomide; Ld,
Ld18 (N = 541)
Attal M, Lauwers-Cances, Marit G, et al: lenalidomide
40
maintenance after stem cell transplantation for multiple
20 myeloma. N Engl J Med 366:1782, 2012; McCarthy P,
Hazard ratio: Owzar K, Hofmeister CC, et al: lenalidomide after stem cell
0 Continuous Ld transplantation for multiple myeloma. N Engl J Med
0 6 12 18 24 30 36 42 48 54 60 vs. MPT, 0.72; 366:1770, 2012; Palumbo A, Cavallo F, Gay F, et al: Autolo-
Months P<0.001 gous transplantation and maintenance therapy in multiple
No. at risk: Continuous Ld myeloma. N Engl J Med 371:895, 2014; and Benboubker L,
Continuous Ld 535 400 319 265 218 168 105 55 19 2 0 vs. Ld18, 0.70; Dimopoulos MA, Dispenzieri A, et al: Lenalidomide and
Ld18 541 391 319 265 167 108 56 30 7 2 0 P<0.001 dexamethasone in transplant ineligible patients with myeloma.
MPT 547 380 304 244 170 116 58 28 6 1 0 N Engl J Med 371:906, 2014.)