Page 2018 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2018
Chapter 118 Hemapheresis 1789
28
stroke in donors receiving G-CSF. Splenic enlargement occurs com- Severe toxicity is most commonly experienced by small patients,
monly, and several instances of splenic rupture have been reported. particularly women, when the blood flow rate is rapid and the pro-
Citrate toxicity is a common complication of PB-HSPC collections; cedure is prolonged beyond a few hours. Symptoms correlate inversely
although usually mild and transient, it may be severe and even with the level of ionized calcium. Extremely low concentrations of
life-threatening in some individuals (see previous section). Venous ionized calcium are encountered routinely in therapeutic procedures
access requires large-bore multilumen catheters, and these appear that process more than 15 L of blood. Acute severe hypocalcemia
particularly susceptible to clotting, especially when patients receive leading to fatal cardiac arrhythmia has been reported in patients who
recombinant cytokine stimulation. Hemorrhage, especially in throm- have undergone apheresis. Controlled infusions of 10% calcium
bocytopenic patients, is another potentially severe complication of gluconate or calcium chloride are effective in the management of
central venous catheter placement. these complications. Because metabolism of citrate occurs predomi-
Some patients who have received multiple prior cycles of chemo- nantly in the liver and kidney, patients with conditions affecting these
therapy and a small proportion of normal healthy donors, referred to organs are at increased risk for severe citrate reactions. Studies of
as “poor mobilizers,” fail to respond adequately to mobilization regi- therapeutic plasma exchange procedures and of PB-HSPC donations
mens. In this circumstance, Plerixafor, a reversible CXCR4 antagonist performed with and without calcium gluconate or calcium chloride
that can block the adhesion of HPCs to the bone marrow stroma can infusion show the effectiveness of continuous calcium infusion for
enhance collections by releasing these cells into the circulation. In a the prevention of mild to moderate citrate toxicity (Fig. 118.8). A
randomized controlled trial, the combination of G-CSF and Plerixa- study of plateletpheresis donors documented sustained effects of
for resulted in a significantly higher proportion of patients with citrate infusion on bone metabolism demonstrated by changes in
non-Hodgkin lymphoma achieving their target cell dose in fewer alkaline phosphatase, osteocalcin, parathyroid hormone, and
29
apheresis days. Similar success is achieved with multiple myeloma 1,25-dihydroxyvitamin D levels, suggesting the potential for long-
patients achieving a 4.8-fold increase peripheral blood CD34 cell term effects on bone metabolism in these donors. Magnesium,
count compared with 1.7-fold with G-CSF alone. Emerging evidence another divalent cation, is also bound by citrate. Despite decreases in
indicates that Plerixafor may be administered “just-in-time” to rescue serum ionized magnesium levels to 39% below baseline, clinical
collections for patients who mobilize poorly with G-CSF alone. effects are not typically observed even during large-volume leukapher-
While the optimum circumstances and timing for administration esis procedures. The most severe allergic complications occur when
have not yet been determined, some guidelines recommend addition plasma is used as the replacement solution, and this risk increases
+
6
of Plerixafor for circulating CD34 cell counts fewer than 20 × 10 /L with repeated exposure. Allergic reactions to ethylene oxide, an agent
on two consecutive days accompanied by increasing WBCs. 30 used in the sterilization of plastic disposable equipment, have been
reported. IV diphenhydramine is usually effective in managing
allergic reactions; premedication with steroids and precautions against
COMPLICATIONS OF THERAPEUTIC APHERESIS anaphylaxis may be necessary for sensitized individuals. Atypical
(hypotension and flushing) and anaphylactic reactions have been
Automated apheresis is a minimal-risk procedure for normal healthy reported in patients receiving angiotensin-converting enzyme inhibi-
blood and plasma donors. The current generation of blood cell sepa- tors undergoing different apheresis procedures, including immuno-
rators is remarkably reliable and equipped with sensitive detection adsorption with staphylococcal protein A columns. These medications
and alarm systems to alert the operator to potential problems. Nev- should be discontinued for at least 24 hours before apheresis. Unlike
ertheless, serious morbidity and rare deaths have been associated with
therapeutic procedures. In most reports, deaths are related to either
complications associated with the use of central venous access cath-
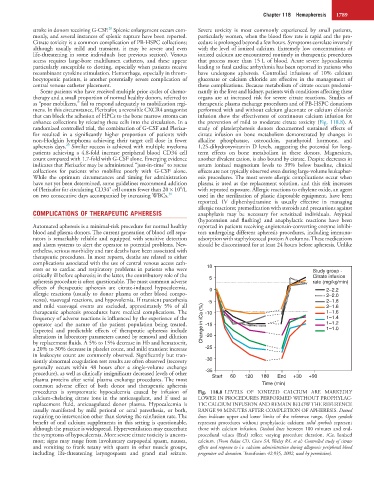
eters or to cardiac and respiratory problems in patients who were 10 Study group -
critically ill before apheresis; in the latter, the contributory role of the 5 Citrate infusion
apheresis procedure is often questionable. The most common adverse rate (mg/kg/min)
effects of therapeutic apheresis are citrate-induced hypocalcemia, 0 2–2.2
allergic reactions (usually to donor plasma or other blood compo- 2–2.0
nents), vasovagal reactions, and hypovolemia. If transient paresthesia -5 2–1.8
and mild vasovagal events are excluded, approximately 5% of all 2–1.6
therapeutic apheresis procedures have medical complications. The -10 1–1.6
frequency of adverse reactions is influenced by the experience of the 1–1.4
operator and the nature of the patient population being treated. Change in iCa (%) -15 1–1.2
Expected and predictable effects of therapeutic apheresis include 1–1.0
alterations in laboratory parameters caused by removal and dilution -20
by replacement fluids. A 5% to 15% decrease in Hb and hematocrit,
a 20% to 30% decrease in platelet count, and mild transient increase -25
in leukocyte count are commonly observed. Significantly but tran-
siently abnormal coagulation test results are often observed (recovery -30
generally occurs within 48 hours after a single-volume exchange -35
procedure), as well as clinically insignificant decreased levels of other Start 60 120 180 End +30 +90
plasma proteins after serial plasma exchange procedures. The most
common adverse effect of both donor and therapeutic apheresis Time (min)
procedures is symptomatic hypocalcemia caused by infusion of Fig. 118.8 LEVELS OF IONIZED CALCIUM ARE MARKEDLY
calcium-chelating citrate ions in the anticoagulant, and if used as LOWER IN PROCEDURES PERFORMED WITHOUT PROPHYLAC-
replacement fluid, anticoagulated donor plasma. Hypocalcemia is TIC CALCIUM INFUSION AND REMAIN BELOW THE REFERENCE
usually manifested by mild perioral or acral paresthesia, or both, RANGE 90 MINUTES AFTER COMPLETION OF APHERESIS. Dotted
requiring no intervention other than slowing the reinfusion rate. The lines indicate upper and lower limits of the reference range. Open symbols
benefit of oral calcium supplements in this setting is questionable, represent procedures without prophylactic calcium; solid symbols represent
although the practice is widespread. Hyperventilation may exacerbate those with calcium infusion. Dashed lines between 180 minutes and end-
the symptoms of hypocalcemia. More severe citrate toxicity is uncom- procedural values (End) reflect varying procedure duration. iCa, Ionized
mon; signs may range from involuntary carpopedal spasm, nausea, calcium. (From Bolan CD, Cecco SA, Wesley RA, et al: Controlled study of citrate
and vomiting to frank tetany with spasm in other muscle groups, effects and response to i.v. calcium administration during allogeneic peripheral blood
including life-threatening laryngospasm and grand mal seizure. progenitor cell donation. Transfusion 42:935, 2002, used by permission).