Page 2014 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2014
Chapter 118 Hemapheresis 1785
to treat these chronic, relapsing, lifelong disorders. Although IBD
respond to a variety of drug regimens, leukapheresis may have a role
as a “steroid-sparing” intervention for patients who develop toxicity 5
from long-term steroid use. Cellsorba removes leukocytes by extra-
corporeal filtration. Granulocyte-monocyte apheresis (Adacolumn)
9
selectively adsorbs cells through columns filled with cellulose beads.
Clinical trials using Adacolumn technology have promising results in
the treatment of ulcerative colitis, with remission rates in excess of
70% compared with conventional medical therapy. These studies
should be interpreted with caution given their high risk of bias and UVA irradiation
the inclusion of predominantly Japanese patients, which may limit 1
how the results apply to populations with different genetic and
10
environmental factors. The optimum course of therapy is yet to be 2
defined. Recent trials have investigated the safety and efficacy of 4
procedures varying from weekly to a daily basis. 11 Whole
blood
Extracorporeal Photopheresis Plasma
Erythrocytes
Photopheresis, although not strictly a “cell removal” procedure, is
considered “apheresis” as it involves automated extracorporeal pho-
tochemotherapy (ECP) treatment that includes leukapheresis, extra-
corporeal photoactivation with 8-methoxypsoralen (8-MOP), a Photomultiplier
light-sensitizing agent, and ex vivo ultraviolet A irradiation. The Buffy module
coat
treated leukocyte fraction (Fig. 118.6) delivering the light-sensitizing Centrifuge
agent directly to the extracorporeal leukocyte fraction avoids any 3
potential toxicity from oral administration of 8-MOP. The mecha-
nism of action of photopheresis is unproved, but may involve induc-
tion of apoptosis of pathogenic T lymphocytes and induction of a
dendritic cell-mediated cytotoxic T-cell response. This therapy has
minimal toxicity and is highly effective in the treatment of patients
with advanced cutaneous T-cell lymphoma. Patients who present
with the erythrodermic disease form and circulating malignant cells 8-Methoxypsoralen
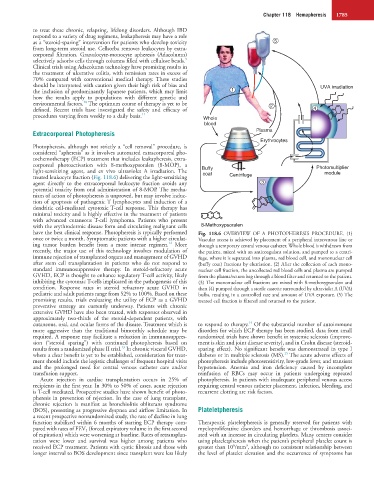
have the best clinical response. Photopheresis is typically performed Fig. 118.6 OVERVIEW OF A PHOTOPHERESIS PROCEDURE. (1)
once or twice a month. Symptomatic patients with a higher circulat- Vascular access is achieved by placement of a peripheral intravenous line or
12
ing tumor burden benefit from a more intense regimen. More through a temporary central venous catheter. Whole blood is withdrawn from
recently, the major use of this technology involves modulation of the patient, mixed with an anticoagulant solution, and pumped to a centri-
immune rejection of transplanted organs and management of GVHD fuge, where it is separated into plasma, red blood cell, and mononuclear cell
after stem cell transplantation in patients who do not respond to (buffy coat) fractions by elutriation. (2) After the collection of each mono-
standard immunosuppressive therapy. In steroid-refractory acute nuclear cell fraction, the uncollected red blood cells and plasma are pumped
GVHD, ECP is thought to enhance regulatory T-cell activity, likely from the plasma/return bag through a blood filter and returned to the patient.
inhibiting the cytotoxic T-cells implicated in the pathogenesis of this (3) The mononuclear cell fractions are mixed with 8-methoxypsoralen and
condition. Response rates in steroid refractory acute GVHD in then (4) pumped through a sterile cassette surrounded by ultraviolet A (UVA)
pediatric and adult patients range from 52% to 100%. Based on these bulbs, resulting in a controlled rate and amount of UVA exposure. (5) The
promising results, trials evaluating the utility of ECP as a GVHD treated cell fraction is filtered and returned to the patient.
preventive strategy are currently underway. Patients with chronic
extensive GVHD have also been treated, with responses observed in
approximately two-thirds of the steroid-dependent patients, with
14
cutaneous, oral, and ocular forms of the disease. Treatment which is to respond to therapy. Of the substantial number of autoimmune
more aggressive than the traditional bimonthly schedule may be disorders for which ECP therapy has been studied, data from small
required. A response may facilitate a reduction in immunosuppres- randomized trials have shown benefit in systemic sclerosis (improve-
sion (“steroid sparing”) with continued photopheresis based on ment is skin and joint disease severity), and in Crohn disease (steroid-
13
results from a randomized phase II trial. In chronic visceral GVHD, sparing effect). No significant benefit was demonstrated in type I
15
where a clear benefit is yet to be established, consideration for treat- diabetes or in multiple sclerosis (MS). The acute adverse effects of
ment should include the logistic challenges of frequent hospital visits photopheresis include photosensitivity, low-grade fever, and transient
and the prolonged need for central venous catheter care and/or hypotension. Anemia and iron deficiency caused by incomplete
transfusion support. reinfusion of RBCs may occur in patients undergoing repeated
Acute rejection in cardiac transplantation occurs in 25% of photopheresis. In patients with inadequate peripheral venous access
recipients in the first year. In 30% to 50% of cases, acute rejection requiring central venous catheter placement, infection, bleeding, and
is T-cell mediated. Prospective studies have shown benefit of photo- recurrent clotting are risk factors.
pheresis in prevention of rejection. In the case of lung transplant,
chronic rejection is manifest as bronchiolitis obliterans syndrome
(BOS), presenting as progressive dyspnea and airflow limitation. In Plateletpheresis
a recent prospective nonrandomized study, the rate of decline in lung
function stabilized within 6 months of starting ECP therapy com- Therapeutic plateletpheresis is generally reserved for patients with
pared with rates of FEV 1 (forced expiratory volume in the first second myeloproliferative disorders and hemorrhage or thrombosis associ-
of expiration) which were worsening at baseline. Rates of retransplan- ated with an increase in circulating platelets. Many centers consider
tation were lower and survival was higher among patients who using plateletpheresis when the patient’s peripheral platelet count is
6
3
received ECP treatment. Patients with cystic fibrosis and those with greater than 10 /mm , although no consistent relationship between
longer interval to BOS development since transplant were less likely the level of platelet elevation and the occurrence of symptoms has