Page 2138 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2138
Chapter 126 Molecular Basis of Blood Coagulation 1899
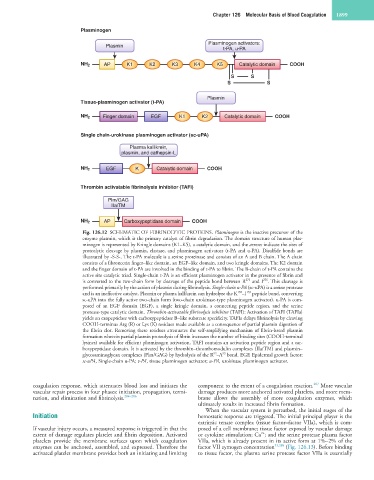
Plasminogen
Plasminogen activators:
Plasmin
t-PA, u-PA
NH 2 AP K1 K2 K3 K4 K5 Catalytic domain COOH
S S
S S
Plasmin
Tissue-plasminogen activator (t-PA)
NH 2 Finger domain EGF K1 K2 Catalytic domain COOH
Single chain-urokinase plasminogen activator (sc-uPA)
Plasma kallikrein,
plasmin, and cathepsin-L
NH 2 EGF K Catalytic domain COOH
Thrombin activatable fibrinolysis inhibitor (TAFI)
Plm/GAG
IIa/TM
NH 2 AP Carboxypeptidase domain COOH
Fig. 126.12 SCHEMATIC OF FIBRINOLYTIC PROTEINS. Plasminogen is the inactive precursor of the
enzyme plasmin, which is the primary catalyst of fibrin degradation. The domain structure of human plas-
minogen is represented by Kringle domains (K1–K5), a catalytic domain, and the arrows indicate the sites of
proteolytic cleavage by plasmin, elastase, and plasminogen activators (t-PA and u-PA). Disulfide bonds are
illustrated by -S-S-. The t-PA molecule is a serine proteinase and consists of an A and B chain. The A chain
consists of a fibronectin finger–like domain, an EGF–like domain, and two kringle domains. The K2 domain
and the finger domain of t-PA are involved in the binding of t-PA to fibrin. The B-chain of t-PA contains the
active site catalytic triad. Single-chain t-PA is an efficient plasminogen activator in the presence of fibrin and
275
is converted to the two-chain form by cleavage of the peptide bond between R and I . This cleavage is
276
performed primarily by the action of plasmin during fibrinolysis. Single-chain u-PA (sc-uPA) is a serine protease
159
158
and is an ineffective catalyst. Plasmin or plasma kallikrein can hydrolyze the K –I peptide bond, converting
sc-uPA into the fully active two-chain form (two-chain urokinase-type plasminogen activator). u-PA is com-
posed of an EGF domain (EGF), a single kringle domain, a connecting peptide region, and the serine
protease-type catalytic domain. Thrombin-activatable fibrinolysis inhibitor (TAFI): Activation of TAFI (TAFIa)
yields an exopeptidase with carboxypeptidase B–like substrate specificity. TAFIa delays fibrinolysis by cleaving
COOH-terminus Arg (R) or Lys (K) residues made available as a consequence of partial plasmin digestion of
the fibrin clot. Removing these residues attenuates the self-amplifying mechanism of fibrin-based plasmin
formation wherein partial plasmin proteolysis of fibrin increases the number of binding sites (COOH-terminal
lysines) available for efficient plasminogen activation. TAFI contains an activation peptide region and a car-
boxypeptidase domain. It is activated by the thrombin–thrombomodulin complexes (IIa/TM) and plasmin–
92
93
glycosaminoglycan complexes (Plm/GAG) by hydrolysis of the R –A bond. EGF, Epidermal growth factor;
sc-uPA, Single-chain u-PA; t-PA, tissue plasminogen activator; u-PA, urokinase plasminogen activator.
287
coagulation response, which attenuates blood loss and initiates the component to the extent of a coagulation reaction. More vascular
vascular repair process in four phases: initiation, propagation, termi- damage produces more anchored activated platelets, and more mem-
nation, and elimination and fibrinolysis. 284–286 brane allows the assembly of more coagulation enzymes, which
ultimately results in increased fibrin formation.
When the vascular system is perturbed, the initial stages of the
Initiation hemostatic response are triggered. The initial principal player is the
extrinsic tenase complex (tissue factor–factor VIIa), which is com-
If vascular injury occurs, a measured response is triggered in that the posed of a cell membrane; tissue factor exposed by vascular damage
2+
extent of damage regulates platelet and fibrin deposition. Activated or cytokine stimulation; Ca ; and the serine protease plasma factor
platelets provide the membrane surfaces upon which coagulation VIIa, which is already present in its active form at 1%–2% of the
enzymes can be anchored, assembled, and expressed. Therefore the factor VII zymogen concentration 53,288 (Fig. 126.13). Before binding
activated platelet membrane provides both an initiating and limiting to tissue factor, the plasma serine protease factor VIIa is essentially