Page 2140 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2140
Chapter 126 Molecular Basis of Blood Coagulation 1901
inert from the catalytic perspective and thus impervious to the The extrinsic tenase complex is under tight supervision by TFPI,
289
abundant protease inhibitors in plasma. Factor VII also competes which can bind both the complex and the product factor Xa (see Fig.
with factor VIIa for tissue factor binding, thus serving as a negative 126.13). 300,301 TFPI, present in low abundance in blood, is released
302
regulator that buffers the overall reaction. 290,291 Factor VII–activating from the vasculature by heparin. If the initiating procoagulant stimu-
protease (FSAP) has also been shown to activate factor VII in the lus is sufficient to overcome the level of this anticoagulant response,
absence of tissue factor. 292–294 The physiologic function of FSAP still a threshold is exceeded and downstream complexes can be formed.
is unclear, but most recently has been suggested to be involved in The limited amounts of factor Xa that escapes inhibition by TFPI
295
inflammation (see the review listed in the References ). The extrinsic and antithrombin bind to available membrane sites and can activate
303
tenase complex (tissue factor–factor VIIa) activates low levels of the tiny amounts of prothrombin to thrombin (see Fig. 126.13). The
zymogens factor X and factor IX to their respective serine protease time period in which factor Xa directly generates picomolar amounts
304
enzymes factor Xa (≈10 pM) and factor IXa (≈1 pM). 296,297 Factor X of thrombin is referred to as the initiation phase of blood coagula-
is the more efficient and abundant substrate. 298,299 tion (Fig. 126.14). During the initiation phase, circulating blood cells
Intravascular Extracellular
fibrinolysis matrix degradation
TAFIa FXIIIa
Crosslinked fibrin
α 2 AP
PAI Plasminogen and
t-PA Plasminogen uPAR cell receptors
t-PA
Plasminogen Pro-MMP
α 2 AP u-PA
Cross linked fibrin PAI
TAFIa α 2 AP
Plasmin
+
Endothelial cell layer
Plasmin
α 2 AP MMP
Crosslinked fibrin
TIMP
Fibrin clot dissolution
ECM degradation
YY DD Legend
DY YXY Enzymes
Fibrin degradation Inhibitors
DXD XY
products
Zymogens
YXD XX Complexes
XXD XD
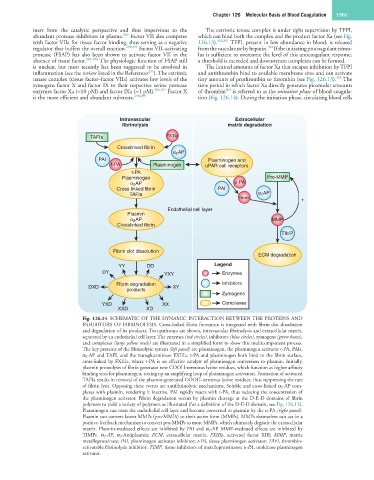
Fig. 126.14 SCHEMATIC OF THE DYNAMIC INTERACTION BETWEEN THE PROTEINS AND
INHIBITORS OF FIBRINOLYSIS. Cross-linked fibrin formation is integrated with fibrin clot dissolution
and degradation of its products. Two pathways are shown, intravascular fibrinolysis and extracellular matrix,
separated by an endothelial cell layer. The enzymes (red circles), inhibitors (blue circles), zymogens (green boxes),
and complexes (large yellow ovals) are illustrated in a simplified form to show this multicomponent process.
The key proteins of the fibrinolytic system (left panel) are plasminogen, the plasminogen activator t-PA, PAIs,
α 2 -AP and TAFI, and the transglutaminase FXIIIa. t-PA and plasminogen both bind to the fibrin surface,
cross-linked by FXIIIa, where t-PA is an effective catalyst of plasminogen conversion to plasmin. Initially,
plasmin proteolysis of fibrin generates new COOH-terminus lysine residues, which function as higher affinity
binding sites for plasminogen, setting up an amplifying loop of plasminogen activation. Formation of activated
TAFIa results in removal of the plasmin-generated COOH-terminus lysine residues, thus suppressing the rate
of fibrin lysis. Opposing these events are antifibrinolytic mechanisms. Soluble and cross-linked α 2 -AP com-
plexes with plasmin, rendering it inactive. PAI rapidly reacts with t-PA, thus reducing the concentration of
the plasminogen activator. Fibrin degradation occurs by plasmin cleavage at the D-E-D domains of fibrin
polymers to yield a variety of polymers as illustrated (for a definition of the D-E-D domain, see Fig. 126.11).
Plasminogen can cross the endothelial cell layer and become converted to plasmin by the u-PA (right panel).
Plasmin can convert latent MMPs (pro-MMPs) to their active form (MMPs). MMPs themselves can act in a
positive-feedback mechanism to convert pro-MMPs to more MMPs, which ultimately degrade the extracellular
matrix. Plasmin-mediated effects are inhibited by PAI and α 2 -AP. MMP-mediated effects are inhibited by
TIMPs. α 2 -AP, α 2 -Antiplasmin; ECM, extracellular matrix; FXIIIa, activated factor XIII; MMP, matrix
metalloproteinase; PAI, plasminogen activator inhibitor; t-PA, tissue plasminogen activator; TAFI, thrombin-
activatable fibrinolysis inhibitor; TIMP, tissue inhibitors of metalloproteinases; u-PA, urokinase plasminogen
activator.