Page 2135 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2135
1896 Part XII Hemostasis and Thrombosis
GPIb-IX-V
Factor V
Fibrinogen
vWF
vWf
PF4
Factor XI “PS”
GPIIb-IIIa
Thrombin
Activation ADP
and Collagen
secretion Epinephrine
Thromboxane A 2
Serotonin “PS”
Factor Va
GPIIb-IIIa
vWF
GPIIb-IIIa
GPIa-IIa
Fibrinogen
Factor Xla
Collagen
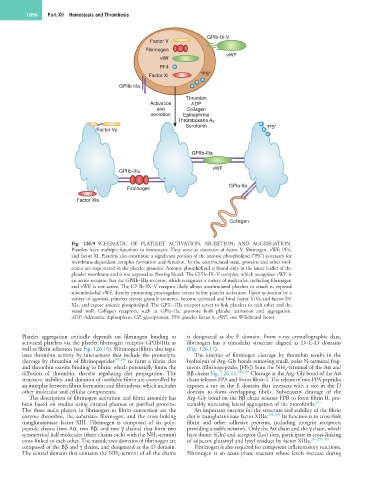
Fig. 126.9 SCHEMATIC OF PLATELET ACTIVATION, SECRETION, AND AGGREGATION.
Platelets have multiple functions in hemostasis. They serve as reservoirs of factor V, fibrinogen, vWF, PF4,
and factor XI. Platelets also contribute a significant portion of the anionic phospholipid (“PS”) necessary for
membrane-dependent complex formation and function. In the unstimulated state, proteins and other mol-
ecules are sequestered in the platelet granules. Anionic phospholipid is found only in the inner leaflet of the
platelet membrane and is not exposed to flowing blood. The GPIb–IX–V complex, which recognizes vWF, is
an active receptor, but the GPIIb–IIIa receptor, which recognizes a variety of molecules, including fibrinogen
and vWF, is not active. The GP Ib–IX–V receptor likely allows unstimulated platelets to attach to exposed
subendothelial vWF, thereby promoting procoagulant events before platelet activation. Upon activation by a
variety of agonists, platelets secrete granule contents, become activated and bind factor V/Va and factor XI/
XIa, and expose anionic phospholipid. The GPII–-IIIa receptor serves to link platelets to each other and the
vessel wall. Collagen receptors, such as GPIa–IIa, promote both platelet activation and aggregation.
ADP, Adenosine diphosphate; GP, glycoprotein; PF4, platelet factor 4; vWF, von Willebrand factor.
Platelet aggregation critically depends on fibrinogen binding to is designated as the E domain. From x-ray crystallographic data,
activated platelets via the platelet fibrinogen receptor GPIIb/IIIa as fibrinogen has a trinodular structure aligned as D–E–D domains
well as fibrin adhesion (see Fig. 126.10). Fibrinogen/fibrin also regu- (Fig. 126.11).
lates thrombin activity by interactions that include the proteolytic The kinetics of fibrinogen cleavage by thrombin results in the
cleavage by thrombin of fibrinopeptides 247,248 to form a fibrin clot hydrolysis of Arg–Gly bonds removing small, polar N-terminal frag-
and thrombin exosite binding to fibrin, which potentially limits the ments (fibrinopeptides [FPs]) from the NH 2 -terminal of the Aα and
diffusion of thrombin, thereby regulating clot propagation. The Bβ chains Fig. 126.11. 249,250 Cleavage at the Arg–Gly bond of the Aα
structure, stability, and duration of insoluble fibrin are controlled by chain releases FPA and forms fibrin I. The release of two FPA peptides
an interplay between fibrin formation and fibrinolysis, which includes exposes a site in the E domain that interacts with a site in the D
other molecular and cellular components. domain to form overlapping fibrils. Subsequent cleavage of the
The description of fibrinogen activation and fibrin assembly has Arg–Gly bond on the Bβ chain releases FPB to form fibrin II, pre-
been based on studies using citrated plasmas or purified proteins. sumably increasing lateral aggregation of the protofibrils. 251
The three main players in fibrinogen to fibrin conversion are the An important enzyme for the structure and stability of the fibrin
enzyme thrombin, the substrates fibrinogen, and the cross-linking clot is transglutaminase factor XIIIa. 252–254 Its function is to cross-link
tranglutaminase factor XIII. Fibrinogen is composed of six poly- fibrin and other adhesive proteins, including integrin receptors,
peptide chains (two Aα, two Bβ, and two γ chains) that form two providing a stable network. Only the Aα chain and the γ chain, which
symmetrical half molecules (three chains each) with the NH 2-termini have donor (Gln) and acceptor (Lys) sites, participate in cross-linking
cross-linked to each other. The outside two domains of fibrinogen are of adjacent glutamyl and lysyl residues by factor XIIIa. 251,255–257
composed of the Bβ and γ chains, and designated as the D domain. Fibrinogen is also required for competent inflammatory reactions.
The central domain that contains the NH 2 -termini of all the chains Fibrinogen is an acute-phase reactant whose levels increase during