Page 2134 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2134
Chapter 126 Molecular Basis of Blood Coagulation 1895
Undisturbed Endothelium
A
Anticoagulant: Thrombin↓ Fibrinolysis↓
PAI-1: Antifibrinolytic Antiplatelet:
TFPI NO
Aggregation Prostacyclin
HCII
AT
Protein S Activation
Heparan sulfate TM Dermatan sulfate ectoADPase
Endothelium
vWf
Disturbed Endothelium
B Fibrinolysis: ↑↓
Procoagulant: ↑Thrombin PAI-1: Antifibrinolytic ↑ Activated platelets
u-PA, t-PA: Profibrinolytic ↑
P-selectin
“PS” Factor V
Endothelium
TF
TF vWF TF
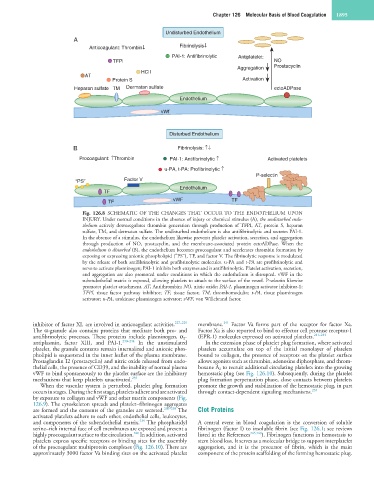
Fig. 126.8 SCHEMATIC OF THE CHANGES THAT OCCUR TO THE ENDOTHELIUM UPON
INJURY. Under normal conditions in the absence of injury or chemical stimulus (A), the undisturbed endo-
thelium actively downregulates thrombin generation through production of TFPI, AT, protein S, heparan
sulfate, TM, and dermatan sulfate. The undisturbed endothelium is also antifibrinolytic and secretes PAI-1.
In the absence of a stimulus, the endothelium likewise prevents platelet activation, secretion, and aggregation
through production of NO, prostacyclin, and the membrane-associated protein ectoADPase. When the
endothelium is disturbed (B), the endothelium becomes procoagulant and accelerates thrombin formation by
exposing or expressing anionic phospholipid (“PS”), TF, and factor V. The fibrinolytic response is modulated
by the release of both antifibrinolytic and profibrinolytic molecules. u-PA and t-PA are profibrinolytic and
serve to activate plasminogen; PAI-1 inhibits both enzymes and is antifibrinolytic. Platelet activation, secretion,
and aggregation are also promoted under conditions in which the endothelium is disrupted. vWF in the
subendothelial matrix is exposed, allowing platelets to attach to the surface of the vessel. P-selectin likewise
promotes platelet attachment. AT, Antithrombin; NO, nitric oxide; PAI-1, plasminogen activator inhibitor-1;
TFPI, tissue factor pathway inhibitor; TF, tissue factor; TM, thrombomodulin; t-PA, tissue plasminogen
activator; u-PA, urokinase plasminogen activator; vWF, von Willebrand factor.
241
inhibitor of factor XI, are involved in anticoagulant activities. 227–229 membrane. Factor Va forms part of the receptor for factor Xa.
The α-granule also contains proteins that meditate both pro- and Factor Xa is also reported to bind to effector cell protease receptor-1
antifibrinolytic processes. These proteins include plasminogen, α 2 - (EPR-1) molecules expressed on activated platelets. 241–244
antiplasmin, factor XIII, and PAI-1. 230–234 In the unstimulated In the extension phase of platelet plug formation, where activated
platelet, the granule contents remain internalized and anionic phos- platelets accumulate on top of the initial monolayer of platelets
pholipid is sequestered in the inner leaflet of the plasma membrane. bound to collagen, the presence of receptors on the platelet surface
Prostaglandin I2 (prostacyclin) and nitric oxide released from endo- allows agonists such as thrombin, adenosine diphosphate, and throm-
thelial cells, the presence of CD39, and the inability of normal plasma boxane A 2 to recruit additional circulating platelets into the growing
vWF to bind spontaneously to the platelet surface are the inhibitory hemostatic plug (see Fig. 126.10). Subsequently, during the platelet
mechanisms that keep platelets unactivated. 235 plug formation perpetuation phase, close contacts between platelets
When the vascular system is perturbed, platelet plug formation promote the growth and stabilization of the hemostatic plug, in part
occurs in stages. During the first stage, platelets adhere and are activated through contact-dependent signaling mechanisms. 235
by exposure to collagen and vWF and other matrix components (Fig.
126.9). The cytoskeleton spreads and platelet–fibrinogen aggregates
are formed and the contents of the granules are secreted. 236–238 The Clot Proteins
activated platelets adhere to each other, endothelial cells, leukocytes,
239
and components of the subendothelial matrix. The phosphatidyl A central event in blood coagulation is the conversion of soluble
serine–rich internal face of cell membranes are exposed and present a fibrinogen (factor I) to insoluble fibrin (see Fig. 126.1; see reviews
240
highly procoagulant surface to the circulation. In addition, activated listed in the References 245,246 ). Fibrinogen functions in hemostasis to
platelets express specific receptors or binding sites for the assembly stem blood loss. It serves as a molecular bridge to support interplatelet
of the procoagulant multiprotein complexes (Fig. 126.10). There are aggregation, and it is the precursor of fibrin, which is the main
approximately 3000 factor Va binding sites on the activated platelet component of the protein scaffolding of the forming hemostatic plug.