Page 2215 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2215
1962 Part XII Hemostasis and Thrombosis
Drug-induced antibody production
Platelet clearance by
phagocytic cells
(See enlargement below)
Ibα Monocyte
B lymphocyte IIb IIb
Ibα IIIa IIIa
Ibβ
IX
V
Ibβ
IX
Platelet
Neoepitope
Drug–glycoprotein complex (ligand-induced bonding site)
e.g., quinine ( ) e.g., eptifibatide ( )
Autoantibody GPIbα, GPIX, GPIIb, and GPIIIa implicated
(e.g., gold)
Platelet activation Drug binds to GPIIb/IIIa
GPV implicated exposing a neoepitope ( )
on GPIIIa
Procoagulant HIT
platelet-derived
microparticles IgG recognizes PF4 ( ) Bound to heparin ( )
FcγIIa receptor ( ) clustering causes platelet activation
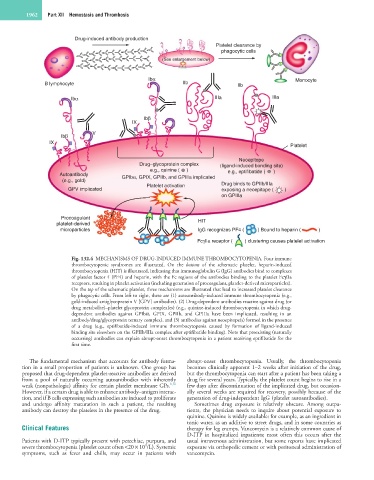
Fig. 132.4 MECHANISMS OF DRUG-INDUCED IMMUNE THROMBOCYTOPENIA. Four immune
thrombocytopenic syndromes are illustrated. On the bottom of the schematic platelet, heparin-induced
thrombocytopenia (HIT) is illustrated, indicating that immunoglobulin G (IgG) antibodies bind to complexes
of platelet factor 4 (PF4) and heparin, with the Fc regions of the antibodies binding to the platelet FcγIIa
receptors, resulting in platelet activation (including generation of procoagulant, platelet-derived microparticles).
On the top of the schematic platelet, three mechanisms are illustrated that lead to increased platelet clearance
by phagocytic cells. From left to right, these are (1) autoantibody-induced immune thrombocytopenia (e.g.,
gold-induced antiglycoprotein V [GPV] antibodies). (2) Drug-dependent antibodies reactive against drug (or
drug metabolite)–platelet glycoprotein complex(es) (e.g., quinine-induced thrombocytopenia in which drug-
dependent antibodies against GPIbα, GPIX, GPIIb, and GPIIIa have been implicated, resulting in an
antibody/drug/glycoprotein ternary complex), and (3) antibodies against neoepitope(s) formed in the presence
of a drug (e.g., eptifibatide-induced immune thrombocytopenia caused by formation of ligand-induced
binding site elsewhere on the GPIIb/IIIa complex after eptifibatide binding). Note that preexisting (naturally
occurring) antibodies can explain abrupt-onset thrombocytopenia in a patient receiving eptifibatide for the
first time.
The fundamental mechanism that accounts for antibody forma- abrupt-onset thrombocytopenia. Usually, the thrombocytopenia
tion in a small proportion of patients is unknown. One group has becomes clinically apparent 1–2 weeks after initiation of the drug,
proposed that drug-dependent platelet-reactive antibodies are derived but the thrombocytopenia can start after a patient has been taking a
from a pool of naturally occurring autoantibodies with inherently drug for several years. Typically, the platelet count begins to rise in a
weak (nonpathologic) affinity for certain platelet membrane GPs. 8,16 few days after discontinuation of the implicated drug, but occasion-
However, if a certain drug is able to enhance antibody–antigen interac- ally several weeks are required for recovery, possibly because of the
tion, and if B cells expressing such antibodies are induced to proliferate generation of drug-independent IgG (platelet autoantibodies).
and undergo affinity maturation in such a patient, the resulting Sometimes drug exposure is relatively obscure. Among outpa-
antibody can destroy the platelets in the presence of the drug. tients, the physician needs to inquire about potential exposure to
quinine. Quinine is widely available: for example, as an ingredient in
tonic water, as an additive to street drugs, and in some countries as
Clinical Features therapy for leg cramps. Vancomycin is a relatively common cause of
D-ITP in hospitalized inpatients; most often this occurs after the
Patients with D-ITP typically present with petechiae, purpura, and usual intravenous administration, but some reports have implicated
9
severe thrombocytopenia (platelet count often <20 × 10 /L). Systemic exposure via orthopedic cement or with peritoneal administration of
symptoms, such as fever and chills, may occur in patients with vancomycin.