Page 2312 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2312
2054 Part XII Hemostasis and Thrombosis
The fully processed protein is then either released into the circulation
or is stored in specialized organelles: the Weibel-Palade bodies (WPBs)
of endothelial cells or the α-granules of platelets.
Dimerization of pro-VWF monomers occurs in the ER through
the formation of disulfide bonds between CK domain monomers in
a tail-to-tail fashion. The location of these intersubunit disulfide
bonds has been localized to a subset of cysteine residues (Cys2771,
Cys2773, and/or Cys2811). In addition, the signal peptide is cleaved
and most of the intrachain disulphide bonds are formed in the ER.
Only pro-VWF dimers are then transported to the Golgi apparatus,
where they make up the basic building blocks for further multimer-
ization. Here, the propeptide (D1–D2) is cleaved by a propeptide
processing protease, likely furin, between amino acids 763–764. The
propeptide continues to be essential for VWF multimerization
because under acidic conditions, it serves as an endogenous chaperone
that promotes additional disulfide bond formation between D3
domains of adjacent dimers in a head-to-head orientation. The
intersubunit disulfide bonds are likely mediated by the alignment of A
the cysteine pairs C1099 and C1142. The VWF subunit is approxi-
mately 250 kDa, whereas resulting disulfide-linked multimers can be
more than 20,000 kDa.
The mature VWF subunit is heavily glycosylated with carbohy-
drate making up approximately 20% of the mass of the mature
subunit. Although the function of these oligosaccharide chains is
largely unknown, they appear to protect VWF from proteolytic
degradation, maintain the multimeric structure of VWF, affect VWF
interaction with platelets and collagen, and influence plasma clear-
ance of VWF. In the ER, 12 N-linked high-mannose-containing
oligosaccharide chains are added to each VWF subunit, and these
appear to be necessary for VWF subunit dimerization. In addition,
the propeptide has three additional potential N-glycosylation sites.
Posttranslational modification continues in the Golgi with the addi-
tion of 10 O-linked oligosaccharides to the peptide chain, and the
sulfation of certain N-linked oligosaccharides, such as Asn384 and
Asn468. Glycan expression is determined by the cell-type. Within
the postGolgi compartment of endothelial cells, the previously added
N-linked glycans undergo further processing with the addition of
ABO groups, as determined by the ABO genotype. Preliminary data B
suggest that there is less N-linked glycosylation in platelets and ABO
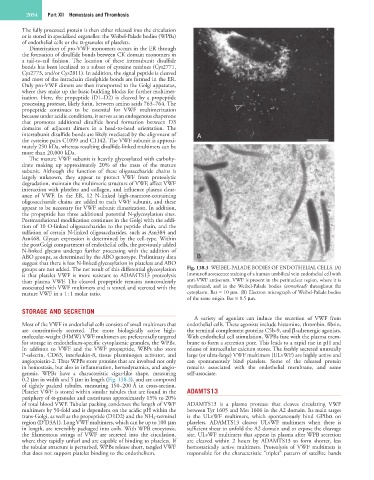
groups are not added. The net result of this differential glycosylation Fig. 138.3 WEIBEL-PALADE BODIES OF ENDOTHELIAL CELLS. (A)
is that platelet VWF is more resistant to ADAMTS13 proteolysis Immunofluorescence staining of a human umbilical vein endothelial cell with
than plasma VWF. The cleaved propeptide remains noncovalently anti-VWF antiserum. VWF is present in the perinuclear region, where it is
associated with VWF multimers and is stored and secreted with the synthesized, and in the Weibel-Palade bodies (arrowhead) throughout the
mature VWF in a 1 : 1 molar ratio. cytoplasm. Bar = 10 µm. (B) Electron micrograph of Weibel-Palade bodies
of the same origin. Bar = 0.5 µm.
STORAGE AND SECRETION
A variety of agonists can induce the secretion of VWF from
Most of the VWF in endothelial cells consists of small multimers that endothelial cells. These agonists include histamine, thrombin, fibrin,
are constitutively secreted. The more biologically active high- the terminal complement proteins C5b-9, and β-adrenergic agonists.
molecular-weight (HMW) VWF multimers are preferentially targeted With endothelial cell stimulation, WPBs fuse with the plasma mem-
for storage in endothelium-specific cytoplasmic granules, the WPBs. brane to form a secretion pore. This leads to a rapid rise in pH and
In addition to VWF and the VWF propeptide, WBPs also store release of intracellular calcium stores. The freshly secreted unusually
P-selectin, CD63, interleukin-8, tissue plasminogen activator, and large (or ultra-large) VWF multimers (ULvWF) are highly active and
angiopoietin-2. Thus WPBs store proteins that are involved not only can spontaneously bind platelets. Some of the released protein
in hemostasis, but also in inflammation, hemodynamics, and angio- remains associated with the endothelial membrane, and some
genesis. WPBs have a characteristic cigar-like shape, measuring self-associate.
0.2 µm in width and 5 µm in length (Fig. 138.3), and are composed
of tightly packed tubules, measuring 150–200 Å in cross-section.
Platelet VWF is stored within similar tubules that are found in the ADAMTS13
periphery of α-granules and constitutes approximately 15% to 20%
of total blood VWF. Tubular packing condenses the length of VWF ADAMTS13 is a plasma protease that cleaves circulating VWF
multimers by 50-fold and is dependent on the acidic pH within the between Tyr 1605 and Met 1606 in the A2 domain. Its main target
trans-Golgi, as well as the propeptide (D1D2) and the NH 2-terminal is the ULvWF multimers, which spontaneously bind GPIbα on
region (D′D3A1). Long VWF multimers, which can be up to 100 µm platelets. ADAMTS13 cleaves ULvWF multimers when there is
in length, are reversibly packaged into coils. With WPB exocytosis, sufficient shear to unfold the A2 domain and to expose the cleavage
the filamentous strings of VWF are secreted into the circulation, site. ULvWF multimers that appear in plasma after WPB secretion
where they rapidly unfurl and are capable of binding to platelets. If are cleaved within 2 hours by ADAMTS13 to form shorter, less
the tubular structure is perturbed, WPBs release short, tangled VWF hemostatically active multimers. Proteolysis of VWF multimers is
that does not support platelet binding to the endothelium. responsible for the characteristic “triplet” pattern of satellite bands