Page 252 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 252
204 Part III Immunologic Basis of Hematology
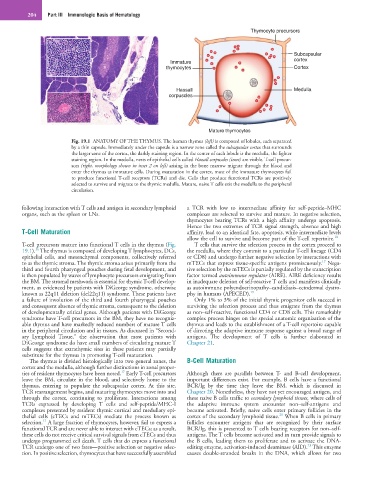
Thymocyte precursors
Subcapsular
Immature cortex
thymocytes Cortex
Hassall Medulla
corpuscles
Mature thymocytes
Fig. 19.1 ANATOMY OF THE THYMUS. The human thymus (left) is composed of lobules, each separated
by a thin capsule. Immediately under the capsule is a narrow zone called the subcapsular cortex that surrounds
the larger zone of the cortex, the darkly staining region. In the center of each lobule is the medulla, the lighter
staining region. In the medulla, nests of epithelial cells called Hassall corpuscles (inset) are visible. T-cell precur-
sors (right, morphology shown in inset 2 on left) arising in the bone marrow migrate through the blood and
enter the thymus as immature cells. During maturation in the cortex, most of the immature thymocytes fail
to produce functional T-cell receptors (TCRs) and die. Cells that produce functional TCRs are positively
selected to survive and migrate to the thymic medulla. Mature, naive T cells exit the medulla to the peripheral
circulation.
following interaction with T cells and antigen in secondary lymphoid a TCR with low to intermediate affinity for self-peptide–MHC
organs, such as the spleen or LNs. complexes are selected to survive and mature. In negative selection,
thymocytes bearing TCRs with a high affinity undergo apoptosis.
Hence the two extremes of TCR signal strength, absence and high
T-Cell Maturation affinity, lead to an identical fate, apoptosis, while intermediate levels
allow the cell to survive and become part of the T-cell repertoire. 18
T-cell precursors mature into functional T cells in the thymus (Fig. T cells that survive the selection process in the cortex proceed to
16
19.1). The thymus is composed of developing T lymphocytes, DCs, the medulla, where they commit to a particular T-cell lineage (CD4
epithelial cells, and mesenchymal components, collectively referred or CD8) and undergo further negative selection by interactions with
17
to as the thymic stroma. The thymic stroma arises primarily from the mTECs that express tissue-specific antigens promiscuously. Nega-
third and fourth pharyngeal pouches during fetal development, and tive selection by the mTECs is partially regulated by the transcription
is then populated by waves of lymphocyte precursors emigrating from factor termed autoimmune regulator (AIRE). AIRE deficiency results
the BM. The stromal meshwork is essential for thymic T-cell develop- in inadequate deletion of self-reactive T cells and manifests clinically
ment, as evidenced by patients with DiGeorge syndrome, otherwise as autoimmune polyendocrinopathy–candidiasis–ectodermal dystro-
known as 22q11 deletion (del22q11) syndrome. These patients have phy in humans (APECED). 19
a failure of involution of the third and fourth pharyngeal pouches Only 1% to 3% of the initial thymic progenitor cells succeed in
and consequent absence of thymic stroma, consequent to the deletion surviving the selection process and thus emigrate from the thymus
of developmentally critical genes. Although patients with DiGeorge as non–self-reactive, functional CD4 or CD8 cells. This remarkably
syndrome have T-cell precursors in the BM, they have no recogniz- complex process hinges on the special anatomic organization of the
able thymus and have markedly reduced numbers of mature T cells thymus and leads to the establishment of a T-cell repertoire capable
in the peripheral circulation and in tissues. As discussed in “Second- of directing the adaptive immune response against a broad range of
ary Lymphoid Tissue,” the observation that most patients with antigens. The development of T cells is further elaborated in
DiGeorge syndrome do have small numbers of circulating mature T Chapter 21.
cells suggests that extrathymic sites in these patients may partially
substitute for the thymus in promoting T-cell maturation.
The thymus is divided histologically into two general zones, the B-Cell Maturation
cortex and the medulla, although further distinctions in zonal proper-
17
ties of resident thymocytes have been noted. Early T-cell precursors Although there are parallels between T- and B-cell development,
leave the BM, circulate in the blood, and selectively home to the important differences exist. For example, B cells have a functional
thymus, entering to populate the subcapsular cortex. At this site, BCR/Ig by the time they leave the BM, which is discussed in
TCR rearrangement begins, and maturing thymocytes move into and Chapter 20. Nonetheless, they have not yet encouraged antigen, and
through the cortex, continuing to proliferate. Interactions among these naive B cells traffic to secondary lymphoid tissues, where cells of
TCRs expressed by developing T cells and self-peptide/MHC-I the adaptive immune system encounter non–self-antigens and
complexes presented by resident thymic cortical and medullary epi- become activated. Briefly, naive cells enter primary follicles in the
20
thelial cells (cTECs and mTECs) mediate the process known as cortex of the secondary lymphoid tissue. When B cells in primary
17
selection. A large fraction of thymocytes, however, fail to express a follicles encounter antigens that are recognized by their surface
functional TCR and are never able to interact with cTECs; as a result, BCR/Ig, this is presented to T cells bearing receptors for non–self-
these cells do not receive critical survival signals from cTECs and thus antigens. The T cells become activated and in turn provide signals to
undergo programmed cell death. T cells that do express a functional the B cells, leading them to proliferate and to activate the DNA-
21
TCR undergo one of two fates—positive selection or negative selec- editing enzyme, activation-induced deaminase (AID). This enzyme
tion. In positive selection, thymocytes that have successfully assembled causes double-stranded breaks in the DNA, which allows for two