Page 416 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 416
Chapter 28 Thrombocytopoiesis 337
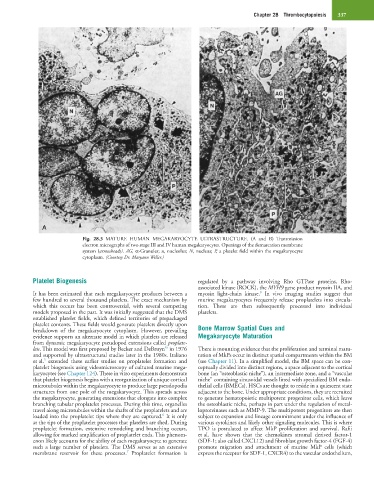
Fig. 28.3 MATURE HUMAN MEGAKARYOCYTE ULTRASTRUCTURE. (A and B) Transmission
electron micrographs of two stage III and IV human megakaryocytes. Openings of the demarcation membrane
system (arrowheads). AG, α-Granules; n, nucleolus; N, nucleus; P, a platelet field within the megakaryocyte
cytoplasm. (Courtesy Dr. Maryann Weller.)
Platelet Biogenesis regulated by a pathway involving Rho GTPase proteins, Rho-
associated kinase (ROCK), the MYH9 gene product myosin IIA, and
8
It has been estimated that each megakaryocyte produces between a myosin light-chain kinase. In vivo imaging studies suggest that
few hundred to several thousand platelets. The exact mechanism by murine megakaryocytes frequently release proplatelets into circula-
which this occurs has been controversial, with several competing tion. These are then subsequently processed into individual
models proposed in the past. It was initially suggested that the DMS platelets.
established platelet fields, which defined territories of prepackaged
platelet contents. These fields would generate platelets directly upon
breakdown of the megakaryocyte cytoplasm. However, prevailing Bone Marrow Spatial Cues and
evidence supports an alternate model in which platelets are released Megakaryocyte Maturation
from dynamic megakaryocyte pseudopod extensions called proplate-
4a
lets. This model was first proposed by Becker and DeBruyn in 1976 There is mounting evidence that the proliferation and terminal matu-
and supported by ultrastructural studies later in the 1980s. Italiano ration of MkPs occur in distinct spatial compartments within the BM
5
et al. extended these earlier studies on proplatelet formation and (see Chapter 11). In a simplified model, the BM space can be con-
platelet biogenesis using videomicroscopy of cultured murine mega- ceptually divided into distinct regions, a space adjacent to the cortical
karyocytes (see Chapter 124). These in vitro experiments demonstrate bone (an “osteoblastic niche”), an intermediate zone, and a “vascular
that platelet biogenesis begins with a reorganization of unique cortical niche” containing sinusoidal vessels lined with specialized BM endo-
microtubules within the megakaryocyte to produce large pseudopodia thelial cells (BMECs). HSCs are thought to reside in a quiescent state
structures from one pole of the megakaryocyte. This spreads across adjacent to the bone. Under appropriate conditions, they are recruited
the megakaryocyte, generating extensions that elongate into complex to generate hematopoietic multipotent progenitor cells, which leave
branching tubular proplatelet processes. During this time, organelles the osteoblastic niche, perhaps in part under the regulation of metal-
travel along microtubules within the shafts of the proplatelets and are loproteinases such as MMP-9. The multipotent progenitors are then
6
loaded into the proplatelet tips where they are captured. It is only subject to expansion and lineage commitment under the influence of
at the tips of the proplatelet processes that platelets are shed. During various cytokines and likely other signaling molecules. This is where
proplatelet formation, extensive remodeling and branching occurs, TPO is postulated to affect MkP proliferation and survival. Rafii
allowing for marked amplification of proplatelet ends. This phenom- et al. have shown that the chemokines stromal derived factor-1
enon likely accounts for the ability of each megakaryocyte to generate (SDF-1; also called CXCL12) and fibroblast growth factor-4 (FGF-4)
such a large number of platelets. The DMS serves as an extensive promote migration and attachment of murine MkP cells (which
7
membrane reservoir for these processes. Proplatelet formation is express the receptor for SDF-1, CXCR4) to the vascular endothelium,